Interferon-gamma
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Interferon-gamma is an immunological agent that is FDA approved for the treatment of serious infections associated with Chronic Granulomatous Disease and delaying time to disease progression in patients with severe, malignant osteopetrosis. Common adverse reactions include Injection site pain, rash, nausea, vomiting, arthralgia, myalgia, headache, fatigue, fever and influenza-like illness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- The recommended dosage of Interferon gamma for the treatment of patients with Chronic Granulomatous Disease and severe, malignant osteopetrosis is 50 mcg/m2 (1 million IU/m2) for patients whose body surface area is greater than 0.5 m2 and 1.5 mcg/kg/dose for patients whose body surface area is equal to or less than 0.5 m2. Note that the above activity is expressed in International Units (1 million IU/50mcg). This is equivalent to what was previously expressed as units (1.5 million U/50mcg). Injections should be administered subcutaneously three times weekly (for example, Monday, Wednesday, Friday). The optimum sites of injection are the right and left deltoid and anterior thigh. Interferon gamma can be administered by a physician, nurse, family member or patient when trained in the administration of subcutaneous injections. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- The formulation does not contain a preservative. A vial of Interferon gamma is suitable for a single use only. The unused portion of any vial should be discarded. Higher doses are not recommended. Safety and efficacy has not been established for Interferon gamma given in doses greater or less than the recommended dose of 50 mcg/m2. The minimum effective dose of Interferon gamma has not been established. Interferon gamma should not be mixed with other drugs in the same syringe.
- Dose Modification: If severe reactions occur, the dosage should be reduced by 50 percent or therapy should be interrupted until the adverse reaction abates.
- Interferon gamma may be administered using either sterilized glass or plastic disposable syringes.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Interferon-gamma in adult patients.
Non–Guideline-Supported Use
Atopic Dermatitis
- Dosage: 0.5 x 10^6 - 1.5 x 10^6 International Units (UI) IM 3 times a week for 12 weeks[1].
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The recommended dosage of Interferon gamma for the treatment of patients with Chronic Granulomatous Disease and severe, malignant osteopetrosis is 50 mcg/m2 (1 million IU/m2) for patients whose body surface area is greater than 0.5 m2 and 1.5 mcg/kg/dose for patients whose body surface area is equal to or less than 0.5 m2. Note that the above activity is expressed in International Units (1 million IU/50mcg). This is equivalent to what was previously expressed as units (1.5 million U/50mcg). Injections should be administered subcutaneously three times weekly (for example, Monday, Wednesday, Friday). The optimum sites of injection are the right and left deltoid and anterior thigh. Interferon gamma can be administered by a physician, nurse, family member or patient when trained in the administration of subcutaneous injections. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
The formulation does not contain a preservative. A vial of Interferon gamma is suitable for a single use only. The unused portion of any vial should be discarded. Higher doses are not recommended. Safety and efficacy has not been established for Interferon gamma given in doses greater or less than the recommended dose of 50 mcg/m2. The minimum effective dose of Interferon gamma has not been established. Interferon gamma should not be mixed with other drugs in the same syringe.
- Dose Modification: If severe reactions occur, the dosage should be reduced by 50 percent or therapy should be interrupted until the adverse reaction abates.
- Interferon gamma may be administered using either sterilized glass or plastic disposable syringes.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Interferon-gamma in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Interferon-gamma in pediatric patients.
Contraindications
- Interferon gamma is contraindicated in patients who develop or have known hypersensitivity to interferon-gamma, E. coli derived products, or any component of the product.
Warnings
Cardiovascular Disorders
- Acute and transient flu-like symptoms such as fever and chills induced by Interferon gamma at doses of 250 mcg/m2/day (greater than 10 times the weekly recommended dose) or higher may exacerbate pre-existing cardiac conditions. Interferon gamma should be used with caution in patients with pre-existing cardiac conditions, including ischemia, congestive heart failure or arrhythmia.
Neurologic Disorders
- Decreased mental status, gait disturbance and dizziness have been observed, particularly in patients receiving Interferon gamma doses greater than 250 mcg/m2/day (greater than 10 times the weekly recommended dose). Most of these abnormalities were mild and reversible within a few days upon dose reduction or discontinuation of therapy. Caution should be exercised when administering Interferon gamma to patients with seizure disorders or compromised central nervous system function.
Bone Marrow Toxicity
- Reversible neutropenia and thrombocytopenia that can be severe and may be dose related have been observed during Interferon gamma therapy. Caution should be exercised when administering Interferon gamma to patients with myelosuppression.
Hepatic Toxicity
- Elevations of AST and/or ALT (up to 25-fold) have been observed during Interferon gamma therapy. The incidence appeared to be higher in patients less than 1 year of age compared to older children. The transaminase elevations were reversible with reduction in dosage or interruption of Interferon gamma treatment. Patients begun on Interferon gamma before age one year should receive monthly assessments of liver function. If severe hepatic enzyme elevations develop, Interferon gamma dosage should be modified.
Adverse Reactions
Clinical Trials Experience
- The following data on adverse reactions are based on the subcutaneous administration of Interferon gamma at a dose of 50 mcg/m2, three times weekly, in patients with Chronic Granulomatous Disease (CGD) during an investigational trial in the United States and Europe. The most common adverse events observed in patients with CGD are shown in the following table:
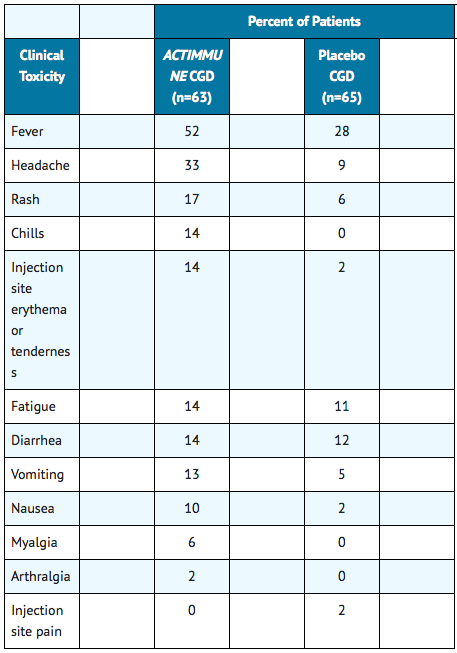
- Miscellaneous adverse events which occurred infrequently in patients with CGD and may have been related to underlying disease included back pain (2 percent versus 0 percent), abdominal pain (8 percent versus 3 percent) and depression (3 percent versus 0 percent) for Interferon gamma and placebo treated patients, respectively. Similar safety data were observed in 34 patients with severe malignant osteopetrosis.
- Interferon gamma has also been evaluated in additional disease states in studies in which patients have generally received higher doses (>100 mcg/m2/three times weekly) administered by intramuscular or subcutaneous injection, or intravenous infusion. All of the previously described adverse reactions which occurred in patients with Chronic Granulomatous Disease have also been observed in patients receiving higher doses. Adverse reactions not observed in patients with Chronic Granulomatous Disease but reported in patients receiving Interferon gamma (Interferon gamma-1b) in other studies include:
Cardiovascular Effects
Central Nervous System Effects
- Confusion
- Disorientation
- Gait disturbance
- Parkinsonian symptoms
- Seizure
- Hallucinations
- Transient ischemic attacks
Gastrointestinal
- Dyspepsia
- Hepatic insufficiency
- Gastrointestinal bleeding
- Pancreatitis, including pancreatitis with fatal outcome.
General Disorders and Administration Site Conditions
Hematologic
Immunological
Metabolic
Musculoskeletal
Pulmonary
Renal
Other
- Chest discomfort
- Exacerbation of dermatomyositis.
Abnormal Laboratory Test Values
- Elevations of ALT and AST
- Neutropenia
- Thrombocytopenia and
- Proteinuria
No neutralizing antibodies to Interferon gamma have been detected in any Chronic Granulomatous Disease patients receiving Interferon gamma.
Postmarketing Experience
Children with CGD less than 3 years of age
- Data on the safety and activity of Interferon gamma in 37 patients under the age of 3 years was pooled from four uncontrolled post-marketing studies. The rate of serious infections per patient-year in this uncontrolled group was similar to the rate observed in the Interferon gamma treatment groups in controlled trials. Developmental parameters (height, weight and endocrine maturation) for this uncontrolled group conformed to national normative scales before and during Interferon gamma therapy.
- In 6 of the 10 patients receiving Interferon gamma therapy before age one year 2-fold to 25-fold elevations from baseline of AST and/or ALT were observed. These elevations occurred as early as 7 days after starting treatment. Treatment with Interferon gamma was interrupted in all 6 of these patients and was restarted at a reduced dosage in 4. Liver transaminase values returned to baseline in all patients and transaminase elevation recurred in one patient upon Interferon gamma rechallenge. An 11-fold alkaline phosphatase elevation and hypokalemia in one patient and neutropenia (ANC=525 cells/mm3) in another patient resolved with interruption of Interferon gamma treatment and did not recur with rechallenge.
- In the post-marketing safety database clinically significant adverse events observed during Interferon gamma therapy in children under the age of three years (n=14) included: two cases of hepatomegaly, and one case each of Stevens-Johnson syndrome, granulomatous colitis, urticaria, and atopic dermatitis.
Drug Interactions
- Interactions between Interferon gamma and other drugs have not been fully evaluated. Caution should be exercised when administering Interferon gamma in combination with other potentially myelosuppressive agents.
- Preclinical studies in rodents using species-specific interferon-gamma have demonstrated a decrease in hepatic microsomal cytochrome P-450 concentrations. This could potentially lead to a depression of the hepatic metabolism of certain drugs that utilize this degradative pathway.
Use in Specific Populations
Pregnancy
Teratogenic Effects
- Interferon gamma has shown an increased incidence of abortions in primates when given in doses approximately 100 times the human dose. A study in pregnant primates treated with subcutaneous doses 2-100 times the human dose failed to demonstrate teratogenic activity for Interferon gamma.
- Female mice treated subcutaneously with rmulFN-gamma at 280 times the maximum recommended clinical dose of Interferon gamma from shortly after birth through puberty but not during pregnancy had offspring which exhibited decreased body weight during the lactation period. The clinical significance of this finding observed following treatment of mice with rmulFN-gamma is uncertain.
- There are no adequate and well-controlled studies in pregnant women. Interferon gamma should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Interferon-gamma in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Interferon-gamma during labor and delivery.
Nursing Mothers
- It is not known whether Interferon gamma is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Interferon gamma, a decision should be made whether to discontinue nursing or to discontinue the drug, dependent upon the importance of the drug to the mother.
Pediatric Use
There is no FDA guidance on the use of Interferon-gamma in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Interferon-gamma in geriatric settings.
Gender
There is no FDA guidance on the use of Interferon-gamma with respect to specific gender populations.
Race
There is no FDA guidance on the use of Interferon-gamma with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Interferon-gamma in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Interferon-gamma in patients with hepatic impairment.
Females of Reproductive Potential and Males
Impairment of Fertility
- Female cynomolgus monkeys treated with daily subcutaneous doses of 30 or 150 mcg/kg Interferon gamma (approximately 20 and 100 times the human dose) exhibited irregular menstrual cycles or absence of cyclicity during treatment. Similar findings were not observed in animals treated with 3 mcg/kg Interferon gamma.
- Female mice receiving recombinant murine IFN-gamma (rmulFN-gamma) at 32 times the maximum recommended clinical dose of Interferon gamma for 4 weeks via intramuscular injection exhibited an increased incidence of atretic ovarian follicles.
- Male cynomolgus monkeys treated intravenously for 4 weeks with 8 times the maximum recommended clinical dose of Interferon gamma exhibited decreased spermatogenesis. The impact of this finding on fertility is not known. Male mice receiving rmulFN-gamma at 32 times the maximum recommended clinical dose of Interferon gamma for 4 weeks via intramuscular injection exhibited decreased spermatogenesis.
- Male mice treated subcutaneously with rmuIFN-gamma from shortly after birth through puberty, with 280 times the maximum recommended clinical dose of Interferon gamma exhibited profound yet reversible decreases in sperm counts and fertility, and an increase in the number of abnormal sperm.
- The clinical significance of these findings observed following treatment of mice with rmulFN-gamma is uncertain.
Immunocompromised Patients
There is no FDA guidance one the use of Interferon-gamma in patients who are immunocompromised.
Administration and Monitoring
Administration
How to Prepare
- 1.- Wash your hands thoroughly with soap and water before preparing the medication. This helps prevent infection.
- 2.- Check the date on the Interferon gamma vial to be sure the drug has not expired.
- 3.- Remove the protective plastic cap and wipe the rubber stopper located on top of the Interferon gamma vial with an alcohol swab.
- 4.- Draw air into the syringe by pulling back on the plunger. The amount of air should be equal to the Interferon gamma dose.
- 5.- Remove and save the needle guard. Slowly insert the needle straight through the center of the rubber stopper into the Interferon gamma vial.
- 6.- Gently push the plunger to discharge the air into the vial.
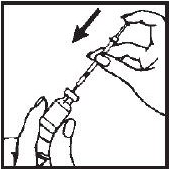
- 7.- Turn the vial upside down with the syringe needle still in it and hold it in one hand. Be sure the tip of the needle is in the solution. Using your other hand slowly pull back on the plunger in a continuous motion until the correct amount of Interferon gamma® solution is in the syringe.
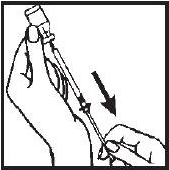
- 8.- Remove the needle from the Interferon gamma vial and replace the needle guard until time of administration or injection. Administration should be as soon after filling the syringe as possible; do not store Interferon gamma in the syringe.

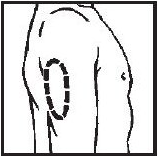
Selecting injection site
- Upper Arm
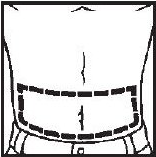
- Abdomen
- Thigh
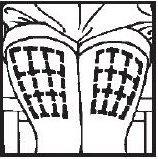
Giving the Medication
- 1.- Cleanse the injection site with an alcohol-saturated cotton ball or cotton swab.
- 2.- Remove the needle guard from the syringe filled with the proper dose of solution and hold the syringe the way you would hold a pencil. Double check that the correct amount of Interferon gamma solution is in the syringe.
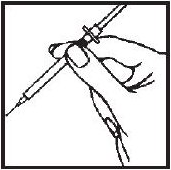
- 3.- Squeeze the skin between your fingers before and during the injection. Insert the needle into the skin at a 45° angle with a quick, firm motion. This hurts less than pushing the needle in slowly.
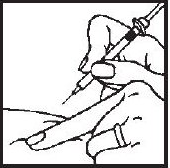
- 4.- After the needle is in, pull back very slightly with one hand on the plunger to see if blood comes into the syringe. This is to be sure that the needle has not entered a blood vessel. If blood does come into the syringe, do not inject the Interferon gamma® solution. Withdraw the needle and insert at another location.
- 5.- If blood does not come into the syringe, slowly (within a few seconds) inject the solution by gently pushing the plunger until the syringe is empty.
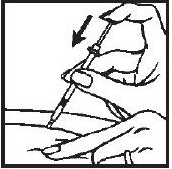
- 6.- Withdraw the needle quickly, pulling it straight out, and apply pressure over the injection site with a dry gauze pad or cotton ball. A drop of blood may appear. Put a Band-Aid® on the injection site if desired.
- 7.- To prevent injury, safely dispose of all used needles and syringes after a single use as instructed by your physician by following these simple steps:
- Place all used needles and syringes in a hard, plastic container with a screw-on cap, or a metal container with a plastic lid, such as a coffee can properly labeled as to content. If a metal container is used, cut a small hole in the plastic lid and tape the lid to the metal container. When the metal container is full, cover the hole with tape and throw it away. If a hard, plastic container is used, always screw the cap on tightly after each use. When the plastic container is full, tape around the cap and throw it away.
- Do not use glass or clear, plastic containers, or any container that will be recycled or returned to a store.
- Always store the container out of the reach of children.
- Please check with your doctor, nurse or pharmacist for other suggestions. There may be special state and local laws that they will discuss with you.
- 8.- Occasionally a problem may develop at the injection site. If you notice any of the following signs or symptoms, contact your doctor or nurse:
- A lump or swelling that doesn't go away
- Bruising that doesn't go away
- Any signs of infection or inflammation at an injection site (pus, persistent redness, surrounding skin that is hot to the touch, persistent pain after the injection)
Monitoring
In addition to those tests normally required for monitoring patients with Chronic Granulomatous Disease and osteopetrosis, the following laboratory tests are recommended for all patients on Interferon gamma (Interferon gamma-1b) therapy prior to the beginning of and at three month intervals during treatment.
- Hematologic tests - including complete blood counts, differential and platelet counts
- Blood chemistries - including renal and liver function tests. In patients less than 1 year of age, liver function tests should be measured monthly.
- Urinalysis
IV Compatibility
There is limited information regarding the compatibility of Interferon-gamma and IV administrations.
Overdosage
Central nervous system adverse reactions including decreased mental status, gait disturbance and dizziness have been observed, particularly in cancer patients receiving doses greater than 100 mcg/m2/day by intravenous or intramuscular administration. These abnormalities were reversible within a few days upon dose reduction or discontinuation of therapy. Reversible neutropenia, elevation of hepatic enzymes and of triglycerides, and thrombocytopenia have also been observed.
Pharmacology
| Template:Px | |
Interferon-gamma
| |
| Systematic (IUPAC) name | |
| Human interferon gamma-1b | |
| Identifiers | |
| CAS number | 98059-61-1 |
| ATC code | L03 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 17145.6 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Interferons bind to specific cell surface receptors and initiate a sequence of intracellular events that lead to the transcription of interferon-stimulated genes. The three major groups of interferons (alpha, beta, gamma) have partially overlapping biological activities that include immunoregulation such as increased resistance to microbial pathogens and inhibition of cell proliferation. Type 1 interferons (alpha and beta) bind to the alpha/beta receptor. Interferon-gamma binds to a different cell surface receptor and is classified as Type 2 interferon. Specific effects of interferon-gamma include the enhancement of the oxidative metabolism of macrophages, antibody dependent cellular cytotoxicity (ADCC), activation of natural killer cells (NK cells) , and the expression of Fc receptors and major histocompatibility antigens.
- Chronic Granulomatous Disease (CGD) is an inherited disorder of leukocyte function caused by defects in the enzyme complex responsible for phagocyte superoxide generation. Interferon gamma does not increase phagocyte superoxide production even in treatment responders.
- In severe, malignant osteopetrosis (an inherited disorder characterized by an osteoclast defect, leading to bone overgrowth, and by deficient phagocyte oxidative metabolism), a treatment-related enhancement of superoxide production by phagocytes was observed. Interferon gamma was found to enhance osteoclast function in vivo.2-4
- In both disorders, the exact mechanism(s) by which Interferon gamma has a treatment effect has not been established. Changes in superoxide levels during Interferon gamma therapy do not predict efficacy and should not be used to assess patient response to therapy.
Structure
There is limited information regarding Interferon-gamma Structure in the drug label.
Pharmacodynamics
Effects in Chronic Granulomatous Disease
- A randomized, double-blind, placebo-controlled study of Interferon gamma (Interferon gamma-1b) in patients with Chronic Granulomatous Disease (CGD), was performed to determine whether Interferon gamma administered subcutaneously on a three times weekly schedule could decrease the incidence of serious infectious episodes and improve existing infectious and inflammatory conditions in patients with Chronic Granulomatous Disease. One hundred twenty-eight eligible patients were enrolled on this study including patients with different patterns of inheritance. Most patients received prophylactic antibiotics. Patients ranged in age from 1 to 44 years with the mean age being 14.6 years. The study was terminated early following demonstration of a highly statistically significant benefit of Interferon gamma therapy compared to placebo with respect to time to serious infection (p=0.0036), the primary endpoint of the investigation. Serious infection was defined as a clinical event requiring hospitalization and the use of parenteral antibiotics. The final analysis provided further support for the primary endpoint (p=0.0006). There was a 67 percent reduction in relative risk of serious infection in patients receiving Interferon gamma (n=63) compared to placebo (n=65). Additional supportive evidence in the number of primary serious infections in the Interferon gamma group (30 on placebo versus 14 on Interferon gamma p=0.002) and the total number and rate of serious infections including recurrent events (56 on placebo versus 20 on Interferon gamma p=<0.0001). Moreover, the length of hospitalization for the treatment of all clinical events provided evidence highly supportive of an Interferon gamma treatment benefit. Placebo patients required three times as many inpatient hospitalization days for treatment of clinical events compared to patients receiving Interferon gamma (1493 versus 497 total days, p=0.02). An Interferon gamma treatment benefit with respect to time to serious infection was consistently demonstrated in all subgroup analyses according to stratification factors, including pattern of inheritance, use of prophylactic antibiotics, as well as age. There was a 67 percent reduction in relative risk of serious infection in patients receiving Interferon gamma compared to placebo across all groups. The beneficial effect of Interferon gamma therapy was observed throughout the entire study, in which the mean duration of Interferon gamma administration was 8.9 months/patient.
Effects in Osteopetrosis
- A controlled, randomized study in patients with severe, malignant osteopetrosis was conducted with Interferon gamma administered subcutaneously three times weekly. Sixteen patients were randomized to receive either Interferon gamma plus calcitriol (n=11), or calcitriol alone (n=5). Patients ranged in age from 1 month to 8 years, mean 1.5 years. Treatment failure was considered to be disease progression as defined by 1) death, 2) significant reduction in hemoglobin or platelet counts, 3) a serious bacterial infection requiring antibiotics, or 4) a 50 dB decrease in hearing or progressive optic atrophy. The median time to disease progression was significantly delayed in the Interferon gamma plus calcitriol arm versus calcitriol alone. In the treatment arm, the median was not reached. Based on the observed data, however, the median time to progression in this arm was at least 165 days versus a median of 65 days in the calcitriol alone arm. In an analysis which combined data from a second study, 19 of 24 patients treated with Interferon gamma plus or minus calcitriol for at least 6 months had reduced trabecular bone volume compared to baseline.
Pharmacokinetics
- The intravenous, intramuscular, and subcutaneous pharmacokinetics of Interferon gamma have been investigated in 24 healthy male subjects following single-dose administration of 100 mcg/m2. Interferon gamma is rapidly cleared after intravenous administration (1.4 liters/minute) and slowly absorbed after intramuscular or subcutaneous injection. After intramuscular or subcutaneous injection, the apparent fraction of dose absorbed was greater than 89%. The mean elimination half-life after intravenous administration of 100 mcg/m2 in healthy male subjects was 38 minutes. The mean elimination half-lives for intramuscular and subcutaneous dosing with 100 mcg/m2 were 2.9 and 5.9 hours, respectively. Peak plasma concentrations, determined by ELISA, occurred approximately 4 hours (1.5 ng/mL) after intramuscular dosing and 7 hours (0.6 ng/mL) after subcutaneous dosing. Multiple dose subcutaneous pharmacokinetic studies were conducted in 38 healthy male subjects. There was no accumulation of Interferon gamma after 12 consecutive daily injections of 100 mcg/m2. Pharmacokinetic studies in patients with Chronic Granulomatous Disease have not been performed.
Trace amounts of interferon-gamma were detected in the urine of squirrel monkeys following intravenous administration of 500 mcg/kg. Interferon-gamma was not detected in the urine of healthy human volunteers following administration of 100 mcg/m2 of Interferon gamma by the intravenous, intramuscular and subcutaneous routes. In vitro perfusion studies utilizing rabbit livers and kidneys demonstrate that these organs are capable of clearing interferon-gamma from perfusate. Studies of the administration of interferon-gamma to nephrectomized mice and squirrel monkeys demonstrate a reduction in clearance of interferon-gamma from blood; however, prior nephrectomy did not prevent elimination.
Nonclinical Toxicology
There is limited information regarding Interferon-gamma Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Interferon-gamma Clinical Studies in the drug label.
How Supplied
- Interferon gamma is supplied in single-use vials. The unused portion of each vial should be disposed of according to state and local regulations as instructed by your physician. If you have any questions, contact your physician.
Storage
- Interferon gamma® (Interferon gamma-1b) must be refrigerated immediately. Refrigerate at 36° to 46° Fahrenheit (2° to 8° Centigrade). DO NOT FREEZE.
Images
Drug Images
{{#ask: Page Name::Interferon-gamma |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
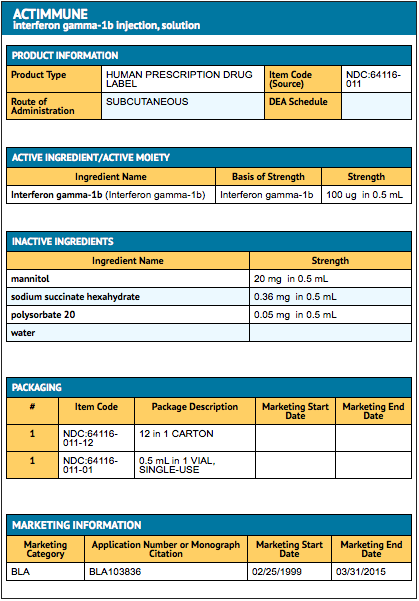
{{#ask: Label Page::Interferon-gamma |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Interferon-gamma Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Interferon-gamma interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Interferon-gamma Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Jang IG, Yang JK, Lee HJ, Yi JY, Kim HO, Kim CW; et al. (2000). "Clinical improvement and immunohistochemical findings in severe atopic dermatitis treated with interferon gamma". J Am Acad Dermatol. 42 (6): 1033–40. PMID 10827410.
{{#subobject:
|Label Page=Interferon-gamma |Label Name=IFNPackage.png
}}
{{#subobject:
|Label Page=Interferon-gamma |Label Name=IFNpackage2.png
}}