GA responsive complex gene transcription laboratory
Editor-In-Chief: Henry A. Hoff
{{free media}}A laboratory is a specialized activity, a construct, you create where you as a student, teacher, or researcher can have hands-on, or as close to hands-on as possible, experience actively analyzing an entity, source, or object of interest. Usually, there's more to do than just analyzing. The construct is often a room, building or institution equipped for scientific research, experimentation as well as analysis.
This laboratory is a continuation of the previous laboratory.
In the room next door is an astronaut on the Mars expedition, three months along on the six-month journey. A physician and lab assistants have been performing tests on her. Because she has been in zero gravity for more than three months her body chemistry and anatomy now differ from what it was in the controlled gravity environment of Earth. She has lost about 10 % each of her bone, muscle, and brain mass. Comparisons with gene expression sequences now and when on Earth have found that the gene expression for alpha-1-B glycoprotein is not normal. If a way to correct this expression cannot be found she must be returned to Earth maybe to recover, maybe not!
But, it is unlikely she will survive three more months at zero g either to be returned to Earth or put on Mars. Worse, the microgravity may not be the only culprit. There is also the radiation of the interplanetary medium.
You have been tasked to examine her DNA to confirm, especially with the extended data between ZNF497 and A1BG, the presence or absence of GA responsive complexes regarding the possible expression of alpha-1-B glycoprotein.
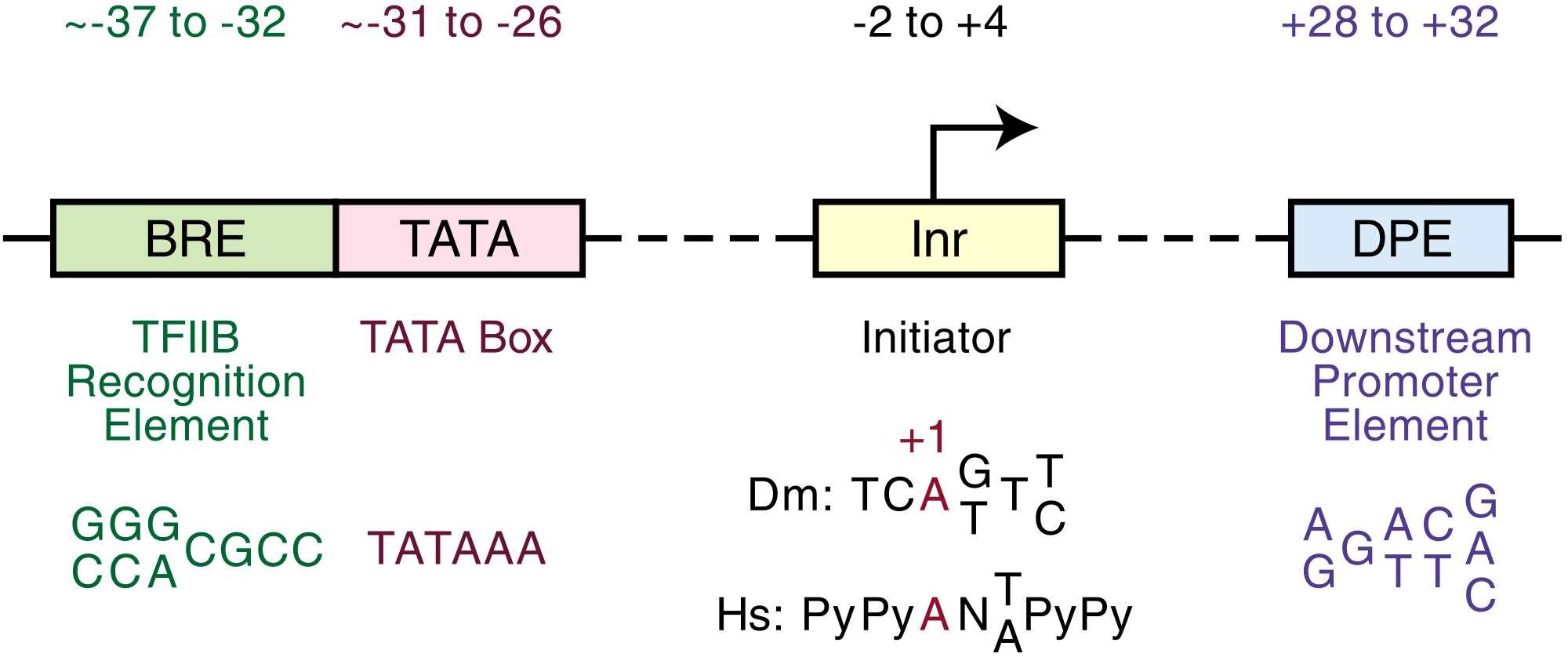
The GA responsive complexes are gene transcription factors (TF), notably the TAACAAA box or GA responsive element (GARE), the pyrimidine box CCTTTT, and the TATCCAC box (Skriver et al., 1991;Gubler and Jacobsen, 1992; Rogers et al., 1994).[1]
GA responsive complexes
{{fairuse}}"Although this GARC [GA responsive complex] may not always be tripartite, most often it includes three sequence motifs, the TAACAAA box or GA responsive element (GARE), the pyrimidine box CCTTTT, and the TATCCAC box (Skriver et al., 1991;Gubler and Jacobsen, 1992; Rogers et al., 1994)."[1]
In the diagram on the right are transient "expression analysis of the relative abilities of the RAmy1A promoters containing mutations to direct expression of the luciferase reporter gene in the rice aleurone."[2]
Each line in the diagram refers to the "5'-upstream regulatory sequence (407 bp long; -380 to +27) from the RAmy1A gene [...] fused to a reporter gene cassette containing the firefly luciferase coding region (luc+) and the 3' terminator from the nopaline synthase gene (Nos-t). The positions of predicted promoter motifs are indicated by open ellipses. Their sequences and positions in the RAmy1A promoter (referred to the transcription initiation site) are indicated. Nucleotide substitutions in the mutated versions are indicated by shaded eclipses."[2]
"The rice WRKY transcription factor OsWRKY71 may be a transcriptional repressor in rice aleurone cells. Transient expression of OsWRKY71 specifically represses GA or OsGAMYB-induced Amy32b α-amylase promoter activity in rice aleurone cells (Washio 2003). Consistent with its role as a transcriptional repressor, OsWRKY71 binds specifically to functionally defined TGAC-containing W boxes of the Amy32b promoter in electrophoretic mobility shift assays (Zhang et al. 2004). Mutations of the two W boxes cause no binding of OsWRKY71 to the promoter, releasing the suppression of OsGAMYB-activated Amy32b expression by OsWRKY71, indicating that OsWRKY71 blocks GA signaling by functionally compromising OsGAMYB (Zhang et al. 2004). Another rice WRKY, namely OsWRKY51, interacts with OsWRKY71, as revealed by bimolecular fluorescence complementation (BiFC) assays, and enhances the binding affinity of OsWRKY71 to W boxes in the Amy32b promoter (Xie et al. 2006)."[3]
GAREs
"In the germinated cereal aleurone layer, gibberellic acids (GA) induce expression of a number of genes encoding hydrolytic enzymes that participate in the mobilization of stored molecules."[2]
"Gibberellins (GAs) are diterpenoid hormones that play crucial roles in plant growth and development, including seed germination, leaf expansion, stem elongation, and flower and fruit development (Hooley, 1994)."[2]
In "the aleurone layer [...] GAs trigger the expression of hydrolytic enzymes through transcription activation of corresponding genes (Jacobsen et al., 1985)."[2]
An "R2R3-type Myb transcription factor GAMYB (Gubler et al., 1995) [...] induces the expression of genes such as high- and low-pI α-amylases, proteinase, and β-glucanase, through direct binding to [the] GA-responsive element (GARE)".[2]
"Raventós et al. (1998) identified a barley (Hordeum vulgare) transcription repressor (HRT) [a barley zinc-finger protein] capable of binding the GARE region of an α-amylase gene and repressing its activity".[2]
"GAMYB is also known to bind the sequence TAACAGAC in vitro, which is the GARE found in barley low-pI α-amylase promoters (Gubler et al., 1995)."[4]
Various "GA-responsive complexes (GARCs) [may] mediate the regulation of GA-regulated gene expression by GAMYB. Several GA-responsive cis-acting elements (GARE) and GARE-like elements (TAACAA/GA, or TAACGTA) have been identified in the promoters of hydrolase genes expressed in the aleurone (Ueguchi-Tanaka et al. 2000; Sutoh and Yamauchi 2003; Washio 2003), expansin genes expressed in internodes (Lee et al. 2001), and many GAMYB-regulated genes expressed in anthers (Tsuji et al. 2006)."[3]
"Although miR159 [a microRNA] has been demonstrated to regulate OsGAMYB expression in anthers, the expression of miR159 is not regulated by GA treatment and the expression is not altered in transgenic lines overexpressing the SLR1 form lacking the DELLA motif (Itoh et al. 2002). In Osgamyb-1 flowers, miR159 expression is not disturbed, suggesting that OsGAMYB is not involved in the regulation of miR159, although a GARE sequence targeted by GAMYB (CAACAAC) is present upstream of the putative cap site of the miR159a transcriptional unit (Tsuji et al. 2006). Thus, miR159 seems not to be regulated by GA signaling."[3]
Pyrimidine boxes
"The pyrimidine box is another promoter element that is observed in cereal GA-responsive promoters examined thus far (Huang et al., 1990). Mutations on the pyrimidine box caused the reduction of the GA induction with a lesser magnitude than those of GARE in the GA-treated aleurone (Gubler and Jacobsen, 1992; Rogers and Rogers, 1992). The pyrimidine box alone could not confer the hormone responsiveness to the minimal promoter of the cauliflower mosaic virus 35S (CaMV35S), suggesting accessory roles of the pyrimidine box on the transcription response to GA (Skriver et al., 1991)."[2]
"[A]leurone proteins that recognized the pyrimidine box sequence [are] from barley (BPBF; Mena et al., 2002) and rice (Oryza sativa; OsDOF3; Washio, 2001)."[2]
"A member of Dof proteins, prolamine box-binding factors PBF that were originally identified to be transcription factors regulating the expression of genes for stored proteins in developing seeds (Vicente-Carbajosa et al., 1997; Mena et al., 1998), BPBF and OsDOF3 are likely to be a pyrimidine box-binding protein in the germinated aleurone."[2]
"OsDOF3, binding the pyrimidine box, affected the DNA binding of GAMYB to GARE".[2]
"Functional promoter analysis using transgenic rice seeds has confirmed that the 5'-regulatory region extending from -232 (nucleotide position relative to the site for transcription initiation) to +31 is sufficient for hormonal regulation by GA (Itoh et al., 1995), along which are found the distal (Pyr-1, -312) and proximal pyrimidine boxes (Pyr-2, -214), GARE (-148), and three potential sites for the Dof binding (D-1, -191; D-2, -109; D-3, -78; [on the diagram in the GA responsive complexes section above]; Huang et al., 1990)."[2]
"The upstream portion from the RAmy1A promoter (-380 to +27) had a stimulatory effect on the GA-induced expression of the reporter gene in transfected aleurone cells."[2]
"The drastic loss of the GA induction associated with a mutation on GARE (M4) further verifies the importance of this motif in the GA-regulated expression of genes. Other mutations of the proximal pyrimidine box (M2) and one site for the Dof binding (M5) also reduced the GA-induced activities to 48% and 54% of the wild-type sequence, respectively, but the effects were not as marked as seen when GARE was mutated."[2]
"Sequence analysis shows that the OsDof3 cDNA encodes a 371-amino acid polypeptide related to the PBF factors of cereal plants. The predicted amino acid sequence of OsDOF3 aligns well with those of maize (Zea mays; Vicente-Carbajosa et al., 1997), wheat (Triticum aestivum), and barley PBF proteins (Mena et al., 1998; [...]). The N-terminal sequence of OsDOF3 contains four Cys residues reminiscent of the Dof zinc finger and shows around 80% sequence identities with PBFs, whereas the C-terminal parts are divergent showing several insertions and deletions."[2]
"Pentanucleotide sequence from the pyrimidine box (CTTTT; Huang et al., 1990) and D-3 (AAAAG) is capable of matching a favored substrate selected by in vitro DNA binding of maize Dof proteins (CTTTT or AAAAG; Yanagisawa and Schmidt, 1999). These results indicate preferential binding of OsDOF3 to two pyrimidine box and D-3 motifs in the RAmy1A promoter context."[2]
"Given the general observations that a couple of the promoter motifs, GARE and the pyrimidine box, always exist in a close distance in the reported GA-responsive promoters (Huang et al., 1990), the synergistic effect on RAmy1A promoter activation are likely to be mediated through protein-protein interaction."[2]
"Some other cis-acting elements, such as pyrimidine boxes (GGTTTT) and TAT boxes (TATCCAT), are usually present in the vicinity of the GARE sequence of genes regulated by GA in cereal aleurone cells (Gubler and Jacobsen 1992; Cercos et al. 1999; Tsuji et al. 2006). For example, GARE and a novel CARE (CAACTC regulatory elements) elements are present in the promoter of rice RAmy1A (Ueguchi-Tanaka et al. 2000; Sutoh and Yamauchi 2003). Cis-element analyses have shown that the OsGAMYB protein activates RAmy1A expression through interaction with GARE in the promoter (Washio 2003). In addition, GARE and CARE are also present in a cysteine proteinase gene REP-1, which is expressed in rice aleurone and is induced by GAs and repressed by ABA. These two elements have been identified as necessary and sufficient for conferring GA inducibility of the REP-1 promoter. Mutations of CARE in the promoters of RAmy1A and REP-1 result in loss of GA inducibility and GAMYB transactivation, suggesting that CARE is a regulatory element for the GA-inducible expression of hydrolase genes in germinating seeds (Sutoh and Yamauchi 2003)."[3]
TATCCAC boxes
"To identify the reason of the low-level expression of BnGID1 in mutant, we sequenced the upstream sequence of BnGID1 and found that there was a GA-responsive complex including GARE (MBS), pyrimidine box and TATC-box, which is same as the upstream sequence of AtGID1a."[5]
W boxes
"Consistent with its role as a transcriptional repressor, OsWRKY71 binds specifically to functionally defined TGAC-containing W boxes of the Amy32b promoter in electrophoretic mobility shift assays (Zhang et al. 2004)."[3]
The "presence of WRKY TF binding sites (C/TTGACC/T, W boxes) in numerous co-regulated Arabidopsis defense gene promoters provided circumstantial evidence that zinc-finger-type WRKY factors play a broad and pivotal role in regulating defenses [10]."[6]
"Plant immune responses are associated with the concerted modulation of a large number of different WRKY transcripts and proteins [15,34–36,37**]. Upon triggering of SA-dependent defenses, at least 49 AtWRKY genes exhibited differential regulation representing separate waves of transcript accumulation or repression [34]. Their promoters are statistically enriched for W boxes, suggesting that they are autoregulated or controlled by other WRKY proteins [34]."[6]
"Details of auto-regulation or cross-regulation by WRKY factors were provided for the parsley group I member PcWRKY1 and its ortholog AtWRKY33 [37**,39,41]. In response to PAMP treatment PcWRKY1 transcripts accumulate rapidly and transiently [42]. AtWRKY33 is activated with similar kinetics by defense-related stimuli [18,34,41]. This rapid response is mediated by a conserved arrangement of three synergistically acting W boxes (WABC). Chromatin immunoprecipitation (ChIP) revealed that in vivo these orthologous W boxes are constitutively occupied by WRKY proteins [37**,41]."[6]
"Some architectural features of the WRKY web are emerging. As motif D containing group I WRKY TFs can be phosphorylated by MAP-kinases, they are likely to serve as the first WRKY proteins activated in response to PAMP-triggered MAPK signaling. Their targets may include the IIe WRKY genes AtWRKY22 and AtWRKY29, which are upregulated by a PAMP-induced MAPK cascade and contain multiple W boxes within their respective promoters [4]."[6]
"The synthesis of SA and the expression of NPR1, a key regulator of some PAMP-triggered responses, appear to be partly controlled by WRKY factors. NPR1 is regulated by WRKY TFs interacting with two W box elements in its 50UTR [44]. Defense-associated SA production is strongly dependent on pathogen-inducible expression of ICS1 [45]. This gene is a likely target of WRKY TFs, as its promoter is enriched for W boxes."[6]
A "missense mutation within its WRKY domain results in conditional activation of defense responses and loss of in vitro binding to W boxes suggesting a negative role of this factor in defense signaling [49]."[6]
The W box is a DNA cis-regulatory element sequence, (T)TGAC(C/T), which is recognized by the family of WRKY transcription factors.[7][8]
Functionality and conservation of the W-box element across plant species shown by gel shift experiments, random binding site selection, yeast one-hybrid screens and co-transfection assays performed with many different WRKY proteins and In silico-based studies have identified clusters of W-boxes in stress-inducible promoters, where the binding of WRKY proteins to W-boxes is a feature of both biotic and abiotic stress responses, together with other plant processes such as germination.[9] It has also been shown that multiple W-boxes have a synergistic effect on transcription.[9]
Almost all WRKY transcription factors bind preferentially to W-boxes, and since their discovery, this has raised the question as to how they show specificity for the promoters of their target genes.[8] Although the W-box core is required, adjacent sequences also play a role in determining binding-site preference.[10] Recent evidence suggests that the TGAC core is more degenerate, composed of a guanine adenine cytosine (GAC) core, and the upstream thymine and downstream pyrimidine flanking sequences help dictate recognition by specific WRKY factors.[11] Basic residues of the WRKY protein domain also are believed to recognize the phosphate backbone of the cis-element.[11]
The solution structure of the C-terminal WRKY domain of Arabidopsis WRKY4 in complex with the W-box DNA has been determined by NMR.[12] A four-stranded β-sheet enters the major groove of DNA in a structure called the β-wedge, where the sheet is nearly perpendicular to the DNA helical ais: as predicted amino acids in the conserved WRKYGQK signature motif contact the W-box DNA.[12]
TAT boxes
"Some other cis-acting elements, such as pyrimidine boxes (GGTTTT) and TAT boxes (TATCCAT), are usually present in the vicinity of the GARE sequence of genes regulated by GA in cereal aleurone cells (Gubler and Jacobsen 1992; Cercos et al. 1999; Tsuji et al. 2006)."[3]
Nucleotide and deduced amino acid of high molecular weight glutenin subunit allele 1Bx23 in common wheat introduced from hexaploid triticale contains the putative TAT box (TATAAAA) and CCACC sequence (CCAATT and CCAT).[13]
"In Proteobacteria this consensus Tat box is best defined as Ser-Arg-Arg-Xaa-Phe-Leu-Lys where the arginine residues are almost invariant, the other amino acids are found at a frequency in excess of 50% and Xaa is a polar amino acid (Berks, 1996; Stanley et al., 2000). The corresponding sequence for plant chloroplast Tat substrates is Arg-Arg-Xaa-Hyd-Leu/Met where Hyd is a hydrophobic amino acid (Peltier et al., 2000). In both cases this Tat or twin-arginine consensus motif is located at the amino-terminal side of the n-region/h-region boundary [...]."[14]
CAREs
The CARE may be called CARE elements.
"GARE and a novel CARE (CAACTC regulatory elements) elements are present in the promoter of rice RAmy1A (Ueguchi-Tanaka et al. 2000; Sutoh and Yamauchi 2003)."[3]
"GARE and CARE are also present in a cysteine proteinase gene REP-1, which is expressed in rice aleurone and is induced by GAs and repressed by ABA. These two elements have been identified as necessary and sufficient for conferring GA inducibility of the REP-1 promoter. Mutations of CARE in the promoters of RAmy1A and REP-1 result in loss of GA inducibility and GAMYB transactivation, suggesting that CARE is a regulatory element for the GA-inducible expression of hydrolase genes in germinating seeds (Sutoh and Yamauchi 2003)."[3]
TATC boxes
These may be the same as TATCCAC boxes.
"Upon cereal seed germination, the seed storage proteins (SSP) and defence proteins (CMe) whose mRNAs are abundant during the maturation phase are silenced. Instead, a different set of genes, encoding hydrolases, is induced in the aleurone upon GA perception. The promoter regions of these genes, such as those encoding α-amylases and proteases (Cejudo et al., 1992; Cercos et al., 1999; Gubler et al., 1995, 1999; Mena et al., 2002), contain the conserved GA response complex (GARC), a tripartite cis motif comprising the GA response element (GARE, 5'-TAACAAA-3'), the pyrimidine box (5'-CTTTT-3') and the TATC box (5'-TATCCAC-3'), all of which are necessary for a full GA response."[15]
Consensus sequences
"Although this GARC [GA responsive complex] may not always be tripartite, most often it includes three sequence motifs, the TAACAAA box or GA responsive element (GARE), the pyrimidine box CCTTTT, and the TATCCAC box (Skriver et al., 1991;Gubler and Jacobsen, 1992; Rogers et al., 1994)."[1]
Nucleotides
DNA mapping has been performed. Her DNA for A1BG promoters can be found at Gene_transcriptions/A1BG#Nucleotides.
Programming
Sample programs for preparing test programs are available at Gene transcriptions/A1BG/Programming.
Hypotheses
- GA responsive complexes are not involved in the transcription of A1BG.
Core promoters
The core promoter is approximately -34 nts upstream from the TSS.
From the first nucleotide just after ZSCAN22 to the first nucleotide just before A1BG are 4460 nucleotides. The core promoter on this side of A1BG extends from approximately 4425 to the possible transcription start site at nucleotide number 4460.
To extend the analysis from inside and just on the other side of ZNF497 some 3340 nts have been added to the data. This would place the core promoter some 3340 nts further away from the other side of ZNF497. The TSS would be at about 4300 nts with the core promoter starting at 4266.
Def. "the factors, including RNA polymerase II itself, that are minimally essential for transcription in vitro from an isolated core promoter" is called the basal machinery, or basal transcription machinery.[17]
Proximal promoters
Def. a "promoter region [juxtaposed to the core promoter that] binds transcription factors that modify the affinity of the core promoter for RNA polymerase.[12][13]"[18] is called a proximal promoter.
The proximal sequence upstream of the gene that tends to contain primary regulatory elements is a proximal promoter.
It is approximately 250 base pairs or nucleotides, nts, upstream of the transcription start site.
The proximal promoter begins about nucleotide number 4210 in the negative direction.
The proximal promoter begins about nucleotide number 4195 in the positive direction.
Distal promoters
The "upstream regions of the human [cytochrome P450 family 11 subfamily A] CYP11A and bovine CYP11B genes [have] a distal promoter in each gene. The distal promoters are located at −1.8 to −1.5 kb in the upstream region of the CYP11A gene and −1.5 to −1.1 kb in the upstream region of the CYP11B gene."[19]
"Using cloned chicken βA-globin genes, either individually or within the natural chromosomal locus, enhancer-dependent transcription is achieved in vitro at a distance of 2 kb with developmentally staged erythroid extracts. This occurs by promoter derepression and is critically dependent upon DNA topology. In the presence of the enhancer, genes must exist in a supercoiled conformation to be actively transcribed, whereas relaxed or linear templates are inactive. Distal protein–protein interactions in vitro may be favored on supercoiled DNA because of topological constraints."[20]
Distal promoter regions may be a relatively small number of nucleotides, fairly close to the TSS such as (-253 to -54)[21] or several regions of different lengths, many nucleotides away, such as (-2732 to -2600) and (-2830 to -2800).[22]
The "[d]istal promoter is not a spacer element."[23]
Using an estimate of 2 knts, a distal promoter to A1BG would be expected after nucleotide number 2460.
Any transcription factor before A1BG from the direction of ZN497 may be out to 2300 nts.
Samplings
Regarding hypothesis 1
GAREs
For the Basic programs (starting with SuccessablesGARE.bas) written to compare nucleotide sequences with the sequences on either the template strand (-), or coding strand (+), of the DNA, in the negative direction (-), or the positive direction (+), including extending the number of nts from 958 to 4445, the programs are, are looking for, and found:
- negative strand in the negative direction (from ZSCAN22 to A1BG) is SuccessablesGARE--.bas, looking for 3'-TAACAAA-5', 0,
- negative strand in the positive direction (from ZNF497 to A1BG) is SuccessablesGARE-+.bas, looking for 3'-TAACAAA-5', 0,
- positive strand in the negative direction is SuccessablesGARE+-.bas, looking for 3'-TAACAAA-5', 0,
- positive strand in the positive direction is SuccessablesGARE++.bas, looking for 3'-TAACAAA-5', 0,
- complement, negative strand, negative direction is SuccessablesGAREc--.bas, looking for 3'-ATTGTTT-5', 0,
- complement, negative strand, positive direction is SuccessablesGAREc-+.bas, looking for 3'-ATTGTTT-5', 0,
- complement, positive strand, negative direction is SuccessablesGAREc+-.bas, looking for 3'-ATTGTTT-5', 0,
- complement, positive strand, positive direction is SuccessablesGAREc++.bas, looking for 3'-ATTGTTT-5', 0,
- inverse complement, negative strand, negative direction is SuccessablesGAREci--.bas, looking for 3'-TTTGTTA-5', 1, 3'-TTTGTTA-5', 230,
- inverse complement, negative strand, positive direction is SuccessablesGAREci-+.bas, looking for 3'-TTTGTTA-5', 0,
- inverse complement, positive strand, negative direction is SuccessablesGAREci+-.bas, looking for 3'-TTTGTTA-5', 0,
- inverse complement, positive strand, positive direction is SuccessablesGAREci++.bas, looking for 3'-TTTGTTA-5', 0,
- inverse, negative strand, negative direction, is SuccessablesGAREi--.bas, looking for 3'-AAACAAT-5', 0,
- inverse, negative strand, positive direction, is SuccessablesGAREi-+.bas, looking for 3'-AAACAAT-5', 0,
- inverse, positive strand, negative direction, is SuccessablesGAREi+-.bas, looking for 3'-AAACAAT-5', 1, 3'-AAACAAT-5', 230,
- inverse, positive strand, positive direction, is SuccessablesGAREi++.bas, looking for 3'-AAACAAT-5', 0.
Pyrimidine boxes
For the Basic programs (starting with SuccessablesPyr.bas) written to compare nucleotide sequences with the sequences on either the template strand (-), or coding strand (+), of the DNA, in the negative direction (-), or the positive direction (+), including extending the number of nts from 958 to 4445, the programs are, are looking for, and found:
- negative strand in the negative direction (from ZSCAN22 to A1BG) is SuccessablesPyr--.bas, looking for 3'-CCTTTT-5', 3, 3'-CCTTTT-5', 2459, 3'-CCTTTT-5', 2927, 3'-CCTTTT-5', 2968,
- negative strand in the positive direction (from ZNF497 to A1BG) is SuccessablesPyr-+.bas, looking for 3'-CCTTTT-5', 1, 3'-CCTTTT-5', 135,
- positive strand in the negative direction is SuccessablesPyr+-.bas, looking for 3'-CCTTTT-5', 0,
- positive strand in the positive direction is SuccessablesPyr++.bas, looking for 3'-CCTTTT-5', 1, 3'-CCTTTT-5', 291,
- complement, negative strand, negative direction is SuccessablesPyrc--.bas, looking for 3'-GGAAAA-5', 0,
- complement, negative strand, positive direction is SuccessablesPyrc-+.bas, looking for 3'-GGAAAA-5', 1, 3'-GGAAAA-5', 291,
- complement, positive strand, negative direction is SuccessablesPyrc+-.bas, looking for 3'-GGAAAA-5', 3, 3'-GGAAAA-5', 2459, 3'-GGAAAA-5', 2927, 3'-GGAAAA-5', 2968,
- complement, positive strand, positive direction is SuccessablesPyrc++.bas, looking for 3'-GGAAAA-5', 1, 3'-GGAAAA-5', 135,
- inverse complement, negative strand, negative direction is SuccessablesPyrci--.bas, looking for 3'-AAAAGG-5', 0,
- inverse complement, negative strand, positive direction is SuccessablesPyrci-+.bas, looking for 3'-AAAAGG-5', 0,
- inverse complement, positive strand, negative direction is SuccessablesPyrci+-.bas, looking for 3'-AAAAGG-5', 4, 3'-AAAAGG-5', 105, 3'-AAAAGG-5', 1107, 3'-AAAAGG-5', 3345, 3'-AAAAGG-5', 3441,
- inverse complement, positive strand, positive direction is SuccessablesPyrci++.bas, looking for 3'-AAAAGG-5', 0,
- inverse, negative strand, negative direction, is SuccessablesPyri--.bas, looking for 3'-TTTTCC-5', 4, 3'-TTTTCC-5', 105, 3'-TTTTCC-5', 1107, 3'-TTTTCC-5', 3345, 3'-TTTTCC-5', 3441,
- inverse, negative strand, positive direction, is SuccessablesPyri-+.bas, looking for 3'-TTTTCC-5', 0,
- inverse, positive strand, negative direction, is SuccessablesPyri+-.bas, looking for 3'-TTTTCC-5', 0,
- inverse, positive strand, positive direction, is SuccessablesPyri++.bas, looking for 3'-TTTTCC-5', 0.
TATCCAC boxes
For the Basic programs (starting with SuccessablesTATC.bas) written to compare nucleotide sequences with the sequences on either the template strand (-), or coding strand (+), of the DNA, in the negative direction (-), or the positive direction (+), including extending the number of nts from 958 to 4445, the programs are, are looking for, and found:
- negative strand in the negative direction (from ZSCAN22 to A1BG) is SuccessablesTATC--.bas, looking for 3'-TATCCAC-5', 0,
- negative strand in the positive direction (from ZNF497 to A1BG) is SuccessablesTATC-+.bas, looking for 3'-TATCCAC-5', 0,
- positive strand in the negative direction is SuccessablesTATC+-.bas, looking for 3'-TATCCAC-5', 0,
- positive strand in the positive direction is SuccessablesTATC++.bas, looking for 3'-TATCCAC-5', 0,
- complement, negative strand, negative direction is SuccessablesTATCc--.bas, looking for 3'-ATAGGTG-5', 0,
- complement, negative strand, positive direction is SuccessablesTATCc-+.bas, looking for 3'-ATAGGTG-5', 0,
- complement, positive strand, negative direction is SuccessablesTATCc+-.bas, looking for 3'-ATAGGTG-5', 0,
- complement, positive strand, positive direction is SuccessablesTATCc++.bas, looking for 3'-ATAGGTG-5', 0,
- inverse complement, negative strand, negative direction is SuccessablesTATCci--.bas, looking for 3'-GTGGATA-5', 0,
- inverse complement, negative strand, positive direction is SuccessablesTATCci-+.bas, looking for 3'-GTGGATA-5', 0,
- inverse complement, positive strand, negative direction is SuccessablesTATCci+-.bas, looking for 3'-GTGGATA-5', 0,
- inverse complement, positive strand, positive direction is SuccessablesTATCci++.bas, looking for 3'-GTGGATA-5', 0,
- inverse, negative strand, negative direction, is SuccessablesTATCi--.bas, looking for 3'-CACCTAT-5', 0,
- inverse, negative strand, positive direction, is SuccessablesTATCi-+.bas, looking for 3'-CACCTAT-5', 0,
- inverse, positive strand, negative direction, is SuccessablesTATCi+-.bas, looking for 3'-CACCTAT-5', 0,
- inverse, positive strand, positive direction, is SuccessablesTATCi++.bas, looking for 3'-CACCTAT-5', 0.
W boxes
For the Basic programs (starting with SuccessablesWbox.bas) written to compare nucleotide sequences with the sequences on either the template strand (-), or coding strand (+), of the DNA, in the negative direction (-), or the positive direction (+), including extending the number of nts from 958 to 4445, the programs are, are looking for, and found:
- negative strand in the negative direction (from ZSCAN22 to A1BG) is SuccessablesWbox--.bas, looking for 3'-(C/T)TGAC(C/T)-5', 5, 3'-CTGACT-5', 17, 3'-TTGACT-5', 130, 3'-TTGACT-5', 307, 3'-CTGACT-5', 1935, 3'-CTGACC-5', 3749,
- negative strand in the positive direction (from ZNF497 to A1BG) is SuccessablesWbox-+.bas, looking for 3'-(C/T)TGAC(C/T)-5', 6, 3'-CTGACC-5', 1662, 3'-CTGACC-5', 2213, 3'-TTGACC-5', 2873, 3'-CTGACT-5', 2945, 3'-TTGACC-5', 4018, 3'-CTGACC-5', 4216,
- positive strand in the negative direction is SuccessablesWbox+-.bas, looking for 3'-(C/T)TGAC(C/T)-5', 1, 3'-CTGACC-5', 734,
- positive strand in the positive direction is SuccessablesWbox++.bas, looking for 3'-(C/T)TGAC(C/T)-5', 3, 3'-TTGACC-5', 1953, 3'-CTGACT-5', 2674, 3'-TTGACT-5', 3735,
- complement, negative strand, negative direction is SuccessablesWboxc--.bas, looking for 3'-(A/G)ACTG(A/G)-5', 1, 3'-GACTGG-5', 734,
- complement, negative strand, positive direction is SuccessablesWboxc-+.bas, looking for 3'-(A/G)ACTG(A/G)-5', 3, 3'-AACTGG-5', 1953, 3'-GACTGA-5', 2674, 3'-AACTGA-5', 3735,
- complement, positive strand, negative direction is SuccessablesWboxc+-.bas, looking for 3'-(A/G)ACTG(A/G)-5', 5, 3'-GACTGA-5', 17, 3'-AACTGA-5', 130, 3'-AACTGA-5', 307, 3'-GACTGA-5', 1935, 3'-GACTGG-5', 3749,
- complement, positive strand, positive direction is SuccessablesWboxc++.bas, looking for 3'-(A/G)ACTG(A/G)-5', 6, 3'-GACTGG-5', 1662, 3'-GACTGG-5', 2213, 3'-AACTGG-5', 2873, 3'-GACTGA-5', 2945, 3'-AACTGG-5', 4018, 3'-GACTGG-5', 4216,
- inverse complement, negative strand, negative direction is SuccessablesWboxci--.bas, looking for 3'-(A/G)GTCA(A/G)-5', 2, 3'-GGTCAG-5', 1353, 3'-GGTCAA-5', 4416,
- inverse complement, negative strand, positive direction is SuccessablesWboxci-+.bas, looking for 3'-(A/G)GTCA(A/G)-5', 6, 3'-AGTCAG-5', 2101, 3'-GGTCAG-5', 2221, 3'-AGTCAG-5', 2608, 3'-AGTCAA-5', 2614, 3'-AGTCAG-5', 2619, 3'-GGTCAG-5', 4270,
- inverse complement, positive strand, negative direction is SuccessablesWboxci+-.bas, looking for 3'-(A/G)GTCA(A/G)-5', 6, 3'-GGTCAG-5', 440, 3'-GGTCAG-5', 577, 3'-GGTCAG-5', 713, 3'-GGTCAG-5', 2249, 3'-GGTCAG-5', 2586, 3'-GGTCAA-5', 4308,
- inverse complement, positive strand, positive direction is SuccessablesWboxci++.bas, looking for 3'-(A/G)GTCA(A/G)-5', 6, 3'-GGTCAG-5', 2025, 3'-AGTCAG-5', 2099, 3'-GGTCAG-5', 2606, 3'-GGTCAG-5', 2997, 3'-GGTCAG-5', 3083, 3'-GGTCAA-5', 3380,
- inverse, negative strand, negative direction, is SuccessablesWboxi--.bas, looking for 3'-(C/T)CAGT(C/T)-5', 6, 3'-CCAGTC-5', 440, 3'-CCAGTC-5', 577, 3'-CCAGTC-5', 713, 3'-CCAGTC-5', 2249, 3'-CCAGTC-5', 2586, 3'-CCAGTT-5', 4308,
- inverse, negative strand, positive direction, is SuccessablesWboxi-+.bas, looking for 3'-(C/T)CAGT(C/T)-5', 6, 3'-CCAGTC-5', 2025, 3'-TCAGTC-5', 2099, 3'-CCAGTC-5', 2606, 3'-CCAGTC-5', 2997, 3'-CCAGTC-5', 3083, 3'-CCAGTT-5', 3380,
- inverse, positive strand, negative direction, is SuccessablesWboxi+-.bas, looking for 3'-(C/T)CAGT(C/T)-5', 2, 3'-CCAGTC-5', 1353, 3'-CCAGTT-5', 4416,
- inverse, positive strand, positive direction, is SuccessablesWboxi++.bas, looking for 3'-(C/T)CAGT(C/T)-5', 6, 3'-TCAGTC-5', 2101, 3'-CCAGTC-5', 2221, 3'-TCAGTC-5', 2608, 3'-TCAGTT-5', 2614, 3'-TCAGTC-5', 2619, 3'-CCAGTC-5', 4270.
TAT boxes
For the Basic programs (starting with SuccessablesTATbox.bas) written to compare nucleotide sequences with the sequences on either the template strand (-), or coding strand (+), of the DNA, in the negative direction (-), or the positive direction (+), including extending the number of nts from 958 to 4445, the programs are, are looking for, and found:
- negative strand in the negative direction (from ZSCAN22 to A1BG) is SuccessablesTATbox--.bas, looking for 3'-TATCCAT-5', 0,
- negative strand in the positive direction (from ZNF497 to A1BG) is SuccessablesTATbox-+.bas, looking for 3'-TATCCAT-5', 0,
- positive strand in the negative direction is SuccessablesTATbox+-.bas, looking for 3'-TATCCAT-5', 0,
- positive strand in the positive direction is SuccessablesTATbox++.bas, looking for 3'-TATCCAT-5', 0,
- complement, negative strand, negative direction is SuccessablesTATboxc--.bas, looking for 3'-ATAGGTA-5', 0,
- complement, negative strand, positive direction is SuccessablesTATboxc-+.bas, looking for 3'-ATAGGTA-5', 0,
- complement, positive strand, negative direction is SuccessablesTATboxc+-.bas, looking for 3'-ATAGGTA-5', 0,
- complement, positive strand, positive direction is SuccessablesTATboxc++.bas, looking for 3'-ATAGGTA-5', 0,
- inverse complement, negative strand, negative direction is SuccessablesTATboxci--.bas, looking for 3'-ATGGATA-5', 1, 3'-ATGGATA-5', 2996,
- inverse complement, negative strand, positive direction is SuccessablesTATboxci-+.bas, looking for 3'-ATGGATA-5', 0,
- inverse complement, positive strand, negative direction is SuccessablesTATboxci+-.bas, looking for 3'-ATGGATA-5', 0,
- inverse complement, positive strand, positive direction is SuccessablesTATboxci++.bas, looking for 3'-ATGGATA-5', 0,
- inverse, negative strand, negative direction, is SuccessablesTATboxi--.bas, looking for 3'-TACCTAT-5', 0,
- inverse, negative strand, positive direction, is SuccessablesTATboxi-+.bas, looking for 3'-TACCTAT-5', 0,
- inverse, positive strand, negative direction, is SuccessablesTATboxi+-.bas, looking for 3'-TACCTAT-5', 1, 3'-TACCTAT-5', 2996,
- inverse, positive strand, positive direction, is SuccessablesTATboxi++.bas, looking for 3'-TACCTAT-5', 0.
CAREs
For the Basic programs (starting with SuccessablesCARE.bas) written to compare nucleotide sequences with the sequences on either the template strand (-), or coding strand (+), of the DNA, in the negative direction (-), or the positive direction (+), including extending the number of nts from 958 to 4445, the programs are, are looking for, and found:
- negative strand in the negative direction (from ZSCAN22 to A1BG) is SuccessablesCARE--.bas, looking for 3'-CAACTC-5', 1, 3'-CAACTC-5', 86,
- negative strand in the positive direction (from ZNF497 to A1BG) is SuccessablesCARE-+.bas, looking for 3'-CAACTC-5', 1, 3'-CAACTC-5', 3292,
- positive strand in the negative direction is SuccessablesCARE+-.bas, looking for 3'-CAACTC-5', 0,
- positive strand in the positive direction is SuccessablesCARE++.bas, looking for 3'-CAACTC-5', 0,
- complement, negative strand, negative direction is SuccessablesCAREc--.bas, looking for 3'-GTTGAG-5', 0,
- complement, negative strand, positive direction is SuccessablesCAREc-+.bas, looking for 3'-GTTGAG-5', 0,
- complement, positive strand, negative direction is SuccessablesCAREc+-.bas, looking for 3'-GTTGAG-5', 1, 3'-GTTGAG-5', 86,
- complement, positive strand, positive direction is SuccessablesCAREc++.bas, looking for 3'-GTTGAG-5', 1, 3'-GTTGAG-5', 3292,
- inverse complement, negative strand, negative direction is SuccessablesCAREci--.bas, looking for 3'-GAGTTG-5', 1, 3'-GAGTTG-5', 1406,
- inverse complement, negative strand, positive direction is SuccessablesCAREci-+.bas, looking for 3'-GAGTTG-5', 0,
- inverse complement, positive strand, negative direction is SuccessablesCAREci+-.bas, looking for 3'-GAGTTG-5', 4, 3'-GAGTTG-5', 2592, 3'-GAGTTG-5', 2704, 3'-GAGTTG-5', 3115, 3'-GAGTTG-5', 4096,
- inverse complement, positive strand, positive direction is SuccessablesCAREci++.bas, looking for 3'-GAGTTG-5', 2, 3'-GAGTTG-5', 1621, 3'-GAGTTG-5', 3290,
- inverse, negative strand, negative direction, is SuccessablesCAREi--.bas, looking for 3'-CTCAAC-5', 4, 3'-CTCAAC-5', 2592, 3'-CTCAAC-5', 2704, 3'-CTCAAC-5', 3115, 3'-CTCAAC-5', 4096,
- inverse, negative strand, positive direction, is SuccessablesCAREi-+.bas, looking for 3'-CTCAAC-5', 2, 3'-CTCAAC-5', 1621, 3'-CTCAAC-5', 3290,
- inverse, positive strand, negative direction, is SuccessablesCAREi+-.bas, looking for 3'-CTCAAC-5', 1, 3'-CTCAAC-5', 1406,
- inverse, positive strand, positive direction, is SuccessablesCAREi++.bas, looking for 3'-CTCAAC-5', 0.
Verifications
To verify that your sampling has explored something, you may need a control group. Perhaps where, when, or without your entity, source, or object may serve.
Another verifier is reproducibility. Can you replicate something about your entity in your laboratory more than 3 times. Five times is usually a beginning number to provide statistics (data) about it.
For an apparent one time or perception event, document or record as much information coincident as possible. Was there a butterfly nearby?
Has anyone else perceived the entity and recorded something about it?
Gene ID: 1, includes the nucleotides between neighboring genes and A1BG. These nucleotides can be loaded into files from either gene toward A1BG, and from template and coding strands. These nucleotide sequences can be found in Gene transcriptions/A1BG. Copying the above discovered CRE boxes and putting the sequences in "⌘F" locates these sequences in the same nucleotide positions as found by the computer programs.
Core promoters GA responsive complexes
From the first nucleotide just after ZSCAN22 to the first nucleotide just before A1BG are 4460 nucleotides. The core promoter on this side of A1BG extends from approximately 4425 to the possible transcription start site at nucleotide number 4460.
From the first nucleotide just after ZNF497 to the first nucleotide just before A1BG are 858 nucleotides. The core promoter on this side of A1BG extends from approximately 824 to the possible transcription start site at nucleotide number 858. Nucleotides (nts) have been added from ZNF497 to A1BG. The TSS for A1BG is now at 4300 nts from just on the other side of ZNF497. The core promoter should now be from 4266 to 4300.
No GARE occur in the core promoters on either side of A1BG.
No Pyrimidine boxes occur in the core promoters on either side of A1BG.
No TATCCAC boxes occur in the core promoters on either side of A1BG.
No W boxes occur in the core promoters on either side of A1BG.
No TAT boxes occur in the core promoters on either side of A1BG.
No CARE occur in the core promoters on either side of A1BG.
Proximal promoter GA responsive complexes
The proximal promoter begins about nucleotide number 4210 in the negative direction.
Inverse W boxes occur within the proximal promoter in the negative direction of A1BG: 3'-GGTCAA-5' at 4416 and 3'-GGTCAA-5' at 4308.
The proximal promoter begins about nucleotide number 4195 in the positive direction.
W boxes occur within the proximal promoter in the positive direction of A1BG: 3'-CTGACC-5' and its complement at 4216 and inverse W boxes occur 3'-GGTCAG-5' and its complement at 4270.
No GARE occur within the proximal promoter on either side of A1BG.
No Pyrimidine boxes occur within the proximal promoter on either side of A1BG.
No TATCCAC boxes occur within the proximal promoter on either side of A1BG.
No TAT boxes occur within the proximal promoter on either side of A1BG.
No CARE occur within the proximal promoter on either side of A1BG.
Distal promoter GA responsive complexes
Using an estimate of 2 knts, a distal promoter to A1BG would be expected after nucleotide number 2460 in the negative direction.
An inverse GARE: 3'-AAACAAT-5' and its complement at 230 occur close to ZSCAN22.
Pyrimidine boxes and their complements: 3'-CCTTTT-5' at 2459, 3'-CCTTTT-5' at 2927, and 3'-CCTTTT-5' at 2968 occur. Inverse pyrimidine boxes and their complements occur 3'-AAAAGG-5' at 105, 3'-AAAAGG-5' at 1107, 3'-AAAAGG-5' at 3345, and 3'-AAAAGG-5' at 3441.
A W box occurs 3'-CTGACC-5' at 3749, whereas 3'-CTGACT-5' at 17, 3'-TTGACT-5' at 130, 3'-TTGACT-5' at 307, and 3'-CTGACC-5' at 734 occur close to ZSCAN22, but 3'-CTGACT-5' at 1935 could be associated ZSCAN22 or an unknown gene between it and A1BG, along with their complements.
W box inverses occur 3'-GGTCAG-5' at 1353 and 3'-AGTCAG-5' at 2101, 3'-GGTCAG-5' at 2221, 3'-AGTCAG-5' at 2608, 3'-AGTCAA-5' at 2614, and 3'-AGTCAG-5' at 2619 along with their complements.
An inverse TAT box occurs 3'-TACCTAT-5' at 2996 with its complement.
A CARE occurs 3'-CAACTC-5' at 86 possibly associated with ZSCAN22. But inverse CARE occur 3'-CTCAAC-5' at 1406, 3'-CTCAAC-5' at 2592, 3'-CTCAAC-5' at 2704, 3'-CTCAAC-5' at 3115, and 3'-CTCAAC-5' at 4096.
A distal promoter to A1BG would be expected after nucleotide 2300 in the positive direction.
A pyrimidine box in the positive direction 3'-CCTTTT-5' at 135 and 3'-CCTTTT-5' at 291 and their complements occur close to ZNF497.
W boxes occur 3'-CTGACC-5' at 1662, 3'-CTGACC-5' at 2213, 3'-TTGACC-5' at 2873, 3'-CTGACT-5' at 2945, and 3'-TTGACC-5' at 4018 that could be associated with A1BG, along with 3'-TTGACC-5' at 1953, 3'-CTGACT-5' at 2674, and 3'-TTGACT-5' at 3735.
No TAT boxes occur in the positive direction.
A CARE occurs 3'-CAACTC-5' at 3292 in the positive direction. But inverse CARE occur 3'-CTCAAC-5' at 1406 and 3'-CTCAAC-5' at 1621 and 3'-CTCAAC-5' at 3290.
No GARE occur on either side of A1BG in the distal promoters.
No TATCCAC boxes occur on either side of A1BG in the distal promoters.
Transcribed GA responsive complexes
Two "distal pyrimidine boxes and a GARE motif are required for the OsGAMYB and RPBF-OsDOF10 (OsDOF3) transcriptional activation of the rice RAmy1A gene promoter (Washio, 2003)."[24]
A Google Scholar search using A1BG and each GA responsive complex TF produced no results.
"The mammalian TRF1 and TRF2 proteins [...] bind double-stranded telomeres via a Myb-like DNA-binding domain and are involved with telomere length regulation and chromosome end protection."[25]
"Telomeres are specialized structures at the end of chromosomes and consist of stretches of repetitive DNA (5'-TTAGGG-3' in vertebrates and trypanosomatids) and associated proteins [5]. Telomeres are essential for maintaining genome stability and cell viability, with dysfunctional telomeres triggering a classic DNA-damage response that enables double-strand breaks and cell cycle arrest [6]."[25]
"In mammals and yeast, telomeric proteins are organized in high order protein complexes known as shelterin or telosome that cap chromosome ends and protect them from fusion or degradation by DNA-repair processes [9, 10, 7]."[25]
"Shelterin/telosome proteins include members or functional homologues of the TRF (TTAGGG repeat-binding factor) or telobox protein family, such as TRF1 and TRF2 from mammals [11] and Tebp1 [12], Taz1 [13] and Tbf1 [14] from yeast. All of these proteins bind double-strand telomeres via a Myb-like DNA-binding domain, which is one of the features that characterize proteins that preferentially bind double-stranded telomeric DNA [15–17]."[25]
"TRF1 can control telomerase access through its interaction with TIN2, PTOP/PIP1 and the single-stranded telomeric DNA-binding protein POT1. TRF1 may also regulates telomerase activity by interacting with PINX1, a natural telomerase inhibitor. In comparison, TRF2 is involved in many functions, including the assembly of the terminal t-loop, negative telomere length regulation and chromosome end protection [18, 11, 16]."[25]
"TRF2 also interacts with enzymes that control G-tail formation, the nucleases XPF1-ERCC1, the MRE11-RAD50-NBS1 (MRN) complex, the RecQ helicase WRN and the 5' exonuclease Apollo [8]. Loss of TRF2 leads to NHEJ-mediated chromosome end-fusion and the accumulation of factors that form the so-called telomere dysfunction-induced foci (TIFs) [21, 22]. Thus, TRF2 can modulate the activity of several enzymes and influence the conformation of telomeric DNA."[25]
Leishmania amazonensis (La) TRF (TTAGGG repeat-binding factor), "LaTRF shared sequence similarities with the canonical Myb-like domain and with the TRFH dimerization domain of human TRF1 and TRF2".[25]
Laboratory reports
Below is an outline for sections of a report, paper, manuscript, log book entry, or lab book entry. You may create your own, of course.
GA responsive complex transcription laboratory
by --Marshallsumter (discuss • contribs) 05:00, 8 November 2018 (UTC)
Abstract
Components of the GA responsive complex which occur in plants have been researched. While a complex of more than one transcription factor does not appear to be operating to transcribe A1BG, one component, the W box can act without other members of the complex being involved. Other GA responsive complex members occur but missing members are usually needed. If they can function in animals with W boxes present to transcribe A1BG, then the hypothesis is false. If only the W box can assist to transcribe A1BG, even though others are present then the hypothesis of GA responsive complex not being involved is true.
Introduction
Many of the transcription factors examined so far could contribute to the transcription of A1BG: AGC boxes (GCC boxes), ATA boxes, C and D boxes, CArG boxes, CRE boxes, Enhancer boxes, Factor II B recognition elements (BREu), HNF6s, HY boxes, Metal responsive elements, and STAT5s.
Gibberellic acids (GA) induce expression of a number of genes. Gibberellins (GAs) are diterpenoid hormones that play crucial roles in plant growth and development. Various GA-responsive complexes (GARCs) may mediate the regulation of GA-regulated gene expression. But, does the GARC or combination of its elements help to transcribe animal growth and development? If so can any affect the transcription of A1BG?
First the components of the GARCs need to be found, then evaluated.
Transcription factors
Many transcription factors (TFs) may occur upstream and occasionally downstream of the transcription start site (TSS), in this gene's promoter. The following have been examined so far: (1) AGC boxes (GCC boxes), (2) ATA boxes, (3) CAAT boxes, (4) C and D boxes, (5) CArG boxes, (6) CENP-B boxes, (7) CGCG boxes, (8) CRE boxes, (9) DREB boxes, (10) EIF4E basal elements (4EBEs), (11) enhancer boxes (E boxes), (12) Factor II B recognition elements, (13) G boxes, (14) GLM boxes, (15) HNF6s, (16) HY boxes, (17) Metal responsive elements (MREs), (18) Motif ten elements (MTEs), (19) STAT5s, (20) TATA boxes, (21) TATCCAC boxes, (22) X boxes and (23) Y boxes.
AGC boxes (GCC boxes)
An AGC box was found in the distal promoter of either gene ZSCAN22 or A1BG on both the template and coding strands. But, as the only known transcription of A1BG occurs between Gene ID: 162968 ZNF497 and Gene ID: 1 A1BG, it is unlikely that this AGC box is naturally used to transcribe A1BG.
A full web search produced several references including a GeneCard[26] for "zinc finger protein 497" and "GCC box", including "May be involved in transcriptional regulation."[26] Zinc fingers are mentioned in association with GCC boxes in plants. It seems unlikely that an AGC box is involved in any way with the transcription of A1BG.
An extension of the nucleotide data for the positive direction from ZNF475 toward A1BG from 958 nts to 4445 nts has not discovered any AGC boxes even in the distal promoter just beyond ZNF497.
ATA boxes
Regarding hypothesis 1: there are no ATA boxes in the core promoter of A1BG from either direction or strand. This hypothesis has been shown to be true. A corollary hypothesis might be 1.1: there are no ATA boxes in the proximal promoter of A1BG from either direction or strand. This corollary hypothesis may be true. "The analysis of the promoter region indicated that a putative ATA box is located 54 nucleotides upstream from the transcription start site".[27] There is one inverse and inverse complement ATA box in the proximal promoter in the positive direction between 4050 and 4300: 3'-AAATAA-5' at 4142, and 3'-TTTATT-5' at 4142. As the TSS is at 4300 nts, this ATA box is some 158 nts away, where with the smaller data set 3'-TTTATT-5' was at 703. As the TSS is at 858 nts, this ATA box is some 155 nts away, which is approximately the same number of nts from the TSS but not close enough to be in the core promoter and not 54 nts upstream from the TSS or to match other such genes with an ATA box.
But the ATA box at 2347 is likely involved in transcription of A1BG in analogy to the rat. Although this has not been confirmed as involved, the existence of this ATA box likely proves hypothesis 1 false.
Regarding hypothesis 2: ATA boxes have a role as downstream signal transducers in A1BG. There is the following inverse ATA box on the negative strand, negative direction: 3'-AAATAA-5' at 4537. On this strand, in this direction the TSS is at 4460 nts from ZSCAN22. This ATA box is 77 nts downstream. So far no published research has been found to verify this type of downstream promoter or enhancer ATA box. There may be another isoform TSS nearby. As such, hypothesis 2 may be true.
Regarding hypothesis 3: ATA boxes may assist transcription of A1BG by other transcription factors. This hypothesis has been shown by literature search to be true. But, none of the ATA boxes for A1BG are close enough to any STAT5 promoter to match known transcription initiation.
CAAT boxes
No CAAT boxes occur on either side of A1BG.
C and D boxes
Regarding hypothesis 1: The C and D boxes are not involved in the transcription of A1BG.
There are no C boxes or D boxes in the core promoter from approximately 4425 to the possible transcription start site at nucleotide number 4460.
There are no C boxes or D boxes in the core promoter from approximately 4266 to the possible transcription start site at nucleotide number 4300.
There are no C boxes or D boxes in the proximal promoter beginning about nucleotide number 4210 in the negative direction.
There is one C box 3'-ACATCA-5' at 4116 but no D boxes in the proximal promoter beginning about nucleotide number 4050 in the positive direction.
There are four C boxes in the distal promoter: 3'-AGTAGT-5' at 2888, 3'-AGTAGT-5' at 2944, 3'-AGTAGT-5' at 3418, and 3'-AGTAGT-5' at 3521 on the negative strand in the negative direction and its complement on the positive strand.
There is one D box in the distal promoter: 3'-AGTCTG-5' at 2947 on the negative strand in the negative direction and its complement on the positive strand.
There is one C box in the distal promoter: 3'-TCATCA-5' at 3251 on the negative strand in the positive direction and its complement on the positive strand.
There is one D box in the distal promoter: 3'-AGTCTG-5' at 3923 on the negative strand in the positive direction and its complement on the positive strand.
Regarding hypothesis 2: If involved they assist transcription by other TFs.
A Google scholar search using key words: "C box", "D box", and A1BG produced zero results.
Regarding hypothesis 3: C and D boxes occur only in the proximal promoter.
GeneID: 60674 GAS5 growth arrest specific 5 (non-protein coding). "This gene produces a spliced long non-coding RNA and is a member of the 5' terminal oligo-pyrimidine class of genes. It is a small nucleolar RNA host gene, containing multiple C/D box snoRNA genes in its introns. Part of the secondary RNA structure of the encoded transcript mimics glucocorticoid response element (GRE) which means it can bind to the DNA binding domain of the glucocorticoid receptor (nuclear receptor subfamily 3, group C, member 1). This action blocks the glucocorticoid receptor from being activated and thereby stops it from regulating the transcription of its target genes. This transcript is also thought to regulate the transcriptional activity of other receptors, such as androgen, progesterone and mineralocorticoid receptors, that can bind to its GRE mimic region. Multiple functions have been associated with this transcript, including cellular growth arrest and apoptosis. It has also been identified as a potential tumor suppressor, with its down-regulation associated with cancer in multiple different tissues."[28]
"The antisense elements located immediately upstream of the D box and/or the D′ box match the sequence of the target RNA, while the areas immediately upstream of the C box and immediately downstream of the D box form a 5′–3′ terminal stem".[29]
"Small nucleolar RNAs (snoRNAs) are noncoding RNAs involved in the processing and modification of ribosomal RNAs. They are grouped in two distinct families, the box C/D family, which catalyzes methylation of 2′-hydroxyls of the pre-rRNA precursor, and the box H/ACA family, which catalyzes the modification of uridines into pseudouridines in various RNAs (reviewed in Refs. [24] and [40])."[30]
"Small nucleolar RNAs (snoRNAs) are 60–300-nucleotide-long RNAs located in the nucleolus or in Cajal bodies. They constitute one of the most abundant classes of ncRNAs [9]. Predominantly intronic, 300 different snoRNA sequences are located in the human genome. They are classified into two categories, those containing boxes C and D; and, those containing boxes H and ACA. snoRNAs are generated after splicing, debranching, and trimming of mRNA introns. Subsequently, mature snoRNAs associate with proteins to form small nucleolar ribonucleoproteins (snoRNPs). These complexes are exported into the nucleolus to participate in rRNA processing [5]."[31]
Tiny "RNAs with a modal length of 18 nt [...] map within -60 to +120 nt of transcription start sites (TSSs) in human, chicken and Drosophila. These transcription initiation RNAs (tiRNAs) are derived from sequences on the same strand as the TSS and are preferentially associated with G+C-rich promoters. The 5' ends of tiRNAs show peak density 10-30 nt downstream of TSSs, indicating that they are processed. tiRNAs are generally, although not exclusively, associated with highly expressed transcripts and sites of RNA polymerase II binding."[32]
"With exception of U3 all box C/D snoRNAs presented in this study are intron-encoded, as it is the general pathway for the biogenesis of this class of snoRNAs (22)."[33]
"Box C/D snoRNAs [...] contain conserved Box C (UGAUGA) and Box D (CUGA) elements located closely to the 5′- and 3′-ends, respectively. Internal copies of these elements are termed Box C′ and Box D′ (20,21)."[33]
Gene ID: 7422 VEGFA vascular endothelial growth factor A. "This gene is a member of the PDGF/VEGF growth factor family. It encodes a heparin-binding protein, which exists as a disulfide-linked homodimer. This growth factor induces proliferation and migration of vascular endothelial cells, and is essential for both physiological and pathological angiogenesis. Disruption of this gene in mice resulted in abnormal embryonic blood vessel formation. This gene is upregulated in many known tumors and its expression is correlated with tumor stage and progression. Elevated levels of this protein are found in patients with POEMS syndrome, also known as Crow-Fukase syndrome. Allelic variants of this gene have been associated with microvascular complications of diabetes 1 (MVCD1) and atherosclerosis. Alternatively spliced transcript variants encoding different isoforms have been described. There is also evidence for alternative translation initiation from upstream non-AUG (CUG) codons resulting in additional isoforms. A recent study showed that a C-terminally extended isoform is produced by use of an alternative in-frame translation termination codon via a stop codon readthrough mechanism, and that this isoform is antiangiogenic. Expression of some isoforms derived from the AUG start codon is regulated by a small upstream open reading frame, which is located within an internal ribosome entry site."[34]
CArG boxes
By combining a literature search with computer analysis of each promoter between ZSCAN22 and A1BG and ZNF497 and A1BG, CArG boxes have been found. To show that these CArG boxes may be used during or for transcription of A1BG at least one transcription factor has been affirmed.
A literature search of more recent results discovered: "Of the [Flowering Locus C] FLC binding sites, 69% contained at least one CArG-box motif with the core consensus sequence CCAAAAAT(G/A)G and an AAA extension at the 3′ end [. Three] other MADS-box flowering-time regulators, SOC1, SVP, and AGAMOUS-LIKE 24 (AGL24), bind to two different CArG-box motifs at 502 bp (CTAAATATGG) and 287 bp (CAATAATTGG) upstream of the translation start in the SEP3 gene (24), consistent with different specificities for the different MADS-box proteins."[35]
These together with the core motif CC(A/T)6GG suggest a more general CArG-box motif of (C(C/A/T)(A/T)6(A/G)G). Subsequent computer-program testing revealed two more general CArG boxes: 3'-CAAAAAAAAG-5' at 1399 nts from ZSCAN22 and 3'-CATTAAAAGG-5' at 3441 nts from ZSCAN22, but none within 4300 nts toward A1BG from ZNF497.
These results show that the presence of CArG boxes on the ZSCAN22 side of A1BG implies their use when transcribing A1BG, although they may be pointing toward ZSCAN22. These suggest that the hypothesis (A1BG is not transcribed by a CArG box) is false. Regarding the second hypothesis (The lack of a CArG box on either side of A1BG does not prove that it is not actively used to transcribe A1BG), the presence of more general CArG boxes in the distal promoter tentatively confirms this hypothesis.
CArG boxes do occur in the distal promoter of A1BG on the ZSCAN22 side only. And, it is likely that a CArG box is involved in some way with the transcription of A1BG.
CENP-B boxes
No CENP-B boxes occur on either side of A1BG.
CGCG boxes
On the negative strand in the negative direction (from ZSCAN22 to A1BG), looking for 3'-(A/C/G)CGCG(C/G/T)-5', there no CGCG boxes in the core promoter.
On the negative strand in the positive direction (from ZNF497 to A1BG), looking for 3'-(A/C/G)CGCG(C/G/T)-5', there no CGCG boxes in the core promoter.
There are no CGCG boxes in the negative direction of the proximal promoter.
There are no CGCG boxes in the positive direction of the other proximal promoter.
There are no CGCG boxes after nucleotide number 2460 in the negative direction of the distal promoter.
There are no CGCG boxes after nucleotide number 2300 in the positive direction of the other distal promoter.
All of the CGCG boxes found are more closely associated with ZSCAN22 or ZNF497 than A1BG.
CRE boxes
There is one CRE box on the negative strand pointing toward A1BG in the proximal promoter in the negative direction between A1BG and ZSCAN22: 3'-TGACGTCA-5' 4317 nts, that can be involved in the transcription of A1BG probably with an Inr rather than a TATA box. This tentatively proves hypothesis 1 false; i.e., A1BG can be transcribed by a CRE box.
DREB boxes
No DREB boxes occur on either side of A1BG.
EIF4E basal elements
No EIF4E basal elements, also eIF4E or (4EBE), occur on either side of A1BG.
Enhancer boxes
The presence of many enhancer boxes on both sides of A1BG demonstrate that the hypothesis: "A1BG is not transcribed by an enhancer box", is false.
The finding by literature search of evidence verifying that at least one transcription factor can enhance or inhibit the transcription of A1BG using one or more enhancer boxes disproves the hypothesis: "Existence of an enhancer box on either side of A1BG does not prove that it is actively used to transcribe A1BG".
Enhancer boxes do occur in the proximal and distal promoters of A1BG. And, it is likely that an enhancer box is involved in some way with the transcription of A1BG.
Factor II B recognition elements
Regarding hypothesis 1: B recognition element (BREu) is not involved in the transcription of A1BG.
In the negative direction, there are no BREs (BREu) in the core promoter from approximately 4425 to the possible transcription start site at nucleotide number 4460.
In the positive direction, there are no BREs (BREu) in the core promoter from approximately 4266 to the possible transcription start site at nucleotide number 4300.
There are no BREs (BREu) in the proximal promoter beginning about nucleotide number 4210 in the negative direction.
There are no BREs (BREu) in the proximal promoter beginning about nucleotide number 4050 in the positive direction.
There is one BREu in the distal promoter: 3'-CCGCACC-5' at 3047 on the negative strand in the negative direction and its complement on the positive strand.
There is one BRE in the distal promoter: 3'-CCGCACC-5' at 2566 on the negative strand in the positive direction and its complement on the positive strand.
Regarding hypothesis 2: If involved it assists transcription by other TFs.
A search of Google Scholar and the full web failed to produce any examples of BREu assisted A1BG transcription.
"A computational study based on statistical analysis of curated promoter sets concluded that up to 25% of human core promoters contain a potential BREu. The motif was found to be enriched in CpG promoters (>30% frequency) but depleted in CpG-less promoters (<10% frequency) [14]."[36]
G boxes
No G boxes occur on either side of A1BG.
GLM boxes
No GLM boxes occur on either side of A1BG.
HNF6s
HNF6s may have a downstream proximal promoter element if the computer nts sampling is additionally, approximately at least 250 nts downstream of the transcription start site. "Downstream" can refer to downstream from an enhancer but before the transcription start site, downstream from a TATA box or an initiator element but before the transcription start site (TSS), downstream from another promoter element and containing the TSS, or downstream after the TSS. The computer programs written to test for HNF6 promoters were limited to 100 nts below the apparent TSSs.
There is a HNF6 on the negative strand in the positive direction (from ZNF497 to A1BG) of 3'-TTCCGGGAA-5' at 808 in the proximal promoter, where the TSS is at 858 nts from ZNF497.
There is no such "downstream" promoter between ZSCAN22 and A1BG.
Both a TATA box or an Inr are within the core promoter. There are no HNF6s within any core promoters per the computer program sampling from ZNF497 or ZSCAN22 and A1BG.
There are no HNF6s within any core promoters per the computer program sampling from ZNF497 or ZSCAN22 and A1BG containing either TSS.
No HNF6s were detected at least to 100 nts downstream of each TSS.
There is a HNF6 on the negative strand in the positive direction (from ZNF497 to A1BG) of 3'-TTCCGGGAA-5' at 808 in the proximal promoter, where the TSS is at 858 nts from ZNF497. This direction is the only confirmed transcription of A1BG; therefore, it is likely A1BG is transcribed using this HNF6 transcription factor.
There are two HNF6s on the negative strand in the negative direction, 3'-AAGCAACTT-5' at 3506 and 3'-AAGGGACTT-5' at 3782. Both of these are in the distal promoter between ZSCAN22 and A1BG.
The only known TSS for A1BG lies at 4300 nts from just beyond ZNF497 toward A1BG. There two HNF6s in the proximal promoter between 4050 and 4300, 3'-TTATTGATTA-5' at 4164 and 3'-TATAATTGTT-5' at 4172, i.e. outside from 4242 (-58) to 4250 (-50). This suggests that HNF6 assists in the transcription of A1BG, but not downstream of the TSS.
{{fairuse}}Both "the 2.3 kb and the 160 bp proximal parts of the a1bg promoter direct sex-specific expression of the reporter gene, and that a negative regulatory element resides in the −1 kb to −160 bp region."[37]
"Computer analysis of the 2.3 kb rat a1bg promoter fragment revealed two putative HNF6 sites and one [hepatic nuclear factor 6] HNF6/HNF3 binding site at −2077/−2069, −69/−61 and −137/−128 respectively [...]."[37]
The "GH-dependent sexually dimorphic expression conveyed by the 2.3 kb a1bg promoter is enhanced by the HNF6/HNF3 site [...]."[37]
"HNF6 bound to the a1bg HNF6 oligonucleotide, but in this case, the mutated oligonucleotide was able to compete for binding when added in large excess [...]. However, [...] the HNF6 binding capacity of the mutated oligonucleotide was clearly reduced. A 20 molar excess of the mutated oligonucleotide had only a marginal effect on the binding of HNF6 [...], whereas a 20 molar excess of unlabelled probe [...] completely abolished binding. Supershift analysis with an HNF6 antibody revealed a complex with a slightly lower mobility than the HNF6 complex [...]. By extending the electrophoresis run and including nuclear extract from hypophysectomized rats, devoid of GH and thereby lacking HNF6 (Lahuna et al. 1997), the two different complexes were clearly visualized. The complex with the lower mobility is most probably due to the binding of HNF3, in analogy with what was shown by Lahuna et al. for the CYP2C12 HNF6 binding site; HNF3 can bind to the site in the absence of HNF6 (Lahuna et al. 1997). [...] HNF6 could bind to their respective site in the a1bg promoter in vitro, and the mutations introduced in respective site abolished binding of the corresponding factor."[37]
The "expression of a −116/−89 deletion construct in which also the HNF6 site was mutated, (−116/−89) delmutHNF6-Luc, [...] the generated luciferase activities were reduced in both sexes [...]. This is in contrast to that mutation/deletion of the sites separately only affected the expression in female livers."[37]
The "−116/−89 region contains a site(s) of importance for the GH-dependent and female-specific expression of the a1bg gene, and that the impact of this region together with the HNF6 site is more complex than mere enhancement of the expression in females."[37]
Following "mutation of the HNF6-binding element, mutHNF6-Luc, the sex-differentiated expression was attenuated due to reduced expression in females. Thus, for a1bg, the sex-related difference in amount of HNF6 is likely to contribute to the sex-differentiated and female characteristic expression."[37]
Nuclear "proteins binding to the a1bg −116/−89 region [are] members of the [nuclear factor 1] NF1 and the [octamer transcription factor] Oct families of transcription factors. NF1 genes are expressed in most adult tissues (Osada et al. 1999). It is not known how NF1 modulates transcriptional activity, and both activation and repression of transcription have been reported (Gronostajski 2000). Cofactors such as CBP/p300 and HDAC have been shown to interact with NF1 proteins suggesting modulation of chromatin structure (Chaudhry et al. 1999). NF1 factors have also been shown to interact directly with the basal transcription machinery as well as with other transcription factors, including Stat5 (Kim & Roeder 1994, Mukhopadhyay et al. 2001) and synergistic effects with HNF4 have been reported (Ulvila et al. 2004). In addition to the HNF6, Stat5 and NF1/Oct sites, the a1bg promoter harbours an imperfect HNF4 site at −51/−39 with two mismatches compared with the HNF4 consensus site. HNF4 is clearly important for the expression of CYP2C12 (Sasaki et al. 1999), however, the −51/−39 region in a1bg was not protected in the footprinting analysis and was therefore not analysed further. Like NF1, Oct proteins have been reported to be involved in activation as well as repression of gene expression (Phillips & Luisi 2000). [...] Moreover, NF1 and Oct-1 have been shown to, reciprocally, facilitate each other’s binding (O’Connor & Bernard 1995, Belikov et al. 2004)."[37]
In the diagram on the right is liver "expression of a1bg-luciferase constructs. (A) Stat5 and HNF6 consensus sequences and corresponding sites in the 2.3 kb a1bg promoter alongside with the used mutations. (B) Female (black bars) and male (open bars) rats [results]."[37]
"Computer analysis of the 2.3 kb rat a1bg promoter fragment revealed [a] HNF6 [site] at [...] −69/−61 [...]."[37]
The murine downstream promoter element is only 11 nts displaced from the human one. This suggests a HNF6 participation in human gene transcription of A1BG.
"Computer analysis of the 2.3 kb rat a1bg promoter fragment revealed two putative HNF6 sites [...] at −2077/−2069 [and] −69/−61 [...]."[37]
There are two HNF6s on the negative strand in the negative direction, 3'-AAGCAACTT-5' at 3506 (-954) and 3'-AAGGGACTT-5' at 3782 (-678) in the distal promoter between ZSCAN22 and A1BG. Although much closer than their likely murine counterparts, they are on the other side of A1BG from the HNF6 site confirming hypothesis 1. If active in humans or murine-like HNF6s occur within or beyond ZNF497 in this distal promoter, then human A1BG is transcribed using HNF6 promoters disproving hypothesis 2.
A Google Scholar search using ZNF497 with HNF6 found no articles discussing HNF6 sites inside or associated with ZNF497. To confirm they exist, a data file going 4300 nts to just beyond ZNF497 has been created and tested for a distal promoter on this side. Distal HNF6s in the positive direction, if they exist, would be inside ZNF497 or beyond, e.g., 3'-ATGTCCATGG-5' at 3581 was found.
Literature search has found that HNF6s assist transcription of A1BG by other transcription factors.[37] The proximal HNF6 promoter is -58 to -50 from A1BG TSS. If another HNF6 promoter is at -2.3 kb, it is about -1.4 kb inside ZNF497 which is 3212 nts long. Per analogy to the rat this would be expected.[37]
Per earlier laboratories transcription factors may occur in the distal promoters on the ZNF497 side of A1BG for
- ATA boxes 3'-AATAAA-5' occurs at 3427,
- CArG boxes,
- Enhancer boxes,
- HY boxes,
- MREs and
- STATs 3'-TTCCATGAA-5' occurs at 128.
The HNF6 promoter on the other side of A1BG (at about +3 kb is way beyond -2.1 through ZNF497 unless the DNA is folded to allow the HNF6 on the ZSCAN22 side to be used in analogy to the HNF6 on the same side as in the rat.[37]
HNF6s have a role as downstream signal transducers in A1BG, where the murine downstream promoter element is only 11 nts displaced from the human one. This suggests a HNF6 participation in human gene transcription of A1BG.
HY boxes
HY boxes were not found in either core promoters or the proximal promoters in either direction. However, HY boxes were found in the distal promoters on both sides of A1BG. No genes are described in the literature so far as transcribed from HY boxes in any distal promoters.
Either A1BG can be transcribed by HY boxes in the distal promoter, or A1BG is not transcribed by HY boxes. As the literature appears absent from a Google Scholar advanced search to confirm possible transcription from distal promoters, wet chemistry experiments are needed to test the possibility.
Metal responsive elements (MREs)
By combining a literature search with computer analysis of the promoter between ZSCAN22 and A1BG and ZNF497 and A1BG, metal responsive elements have been found. Literature search has also discovered at least three post-translational isoforms including the unaltered precursor. Although no metal responsive elements overlap any enhancer boxes in the distal promoter, there are elements in the distal promoter.
"The human genome is estimated to contain 700 zinc-finger genes, which perform many key functions, including regulating transcription. [Four] clusters of zinc-finger genes [occur] on human chromosome 19".[38]
Nearby zinc-fingers on chromosome 19 include ZNF497 (GeneID: 162968), ZNF837 (GeneID: 116412), and ZNF8 (GeneID: 7554).
"In rodents and in humans, about one third of the zinc-finger genes carry the Krüppel-associated box (KRAB), a potent repressor of transcription (Margolin et al. 1994), [...]. There are more than 200 KRAB-containing zinc-finger genes in the human genome, about 40% of which reside on chromosome 19 and show a clustered organization suggesting an evolutionary history of duplication events (Dehal et al. 2001)."[38]
ZNF8 is in cluster V along with A1BG.[38]
"In contrast to the four clusters considered [I through IV], one that occurs at the telomere of chromosome 19, which we will call cluster V, has been very stable [over mouse, rat, and human]."[38]
"Apart from the somewhat unexpected location of Zfp35 on mouse chromosome 18 and of the AIBG orthologs on mouse chromosome 15 and rat chromosome 7, there has been little rearrangement."[38]
So far no article has reported any linkage between zinc, including various zinc fingers, or cadmium, and A1BG.
Regarding additional isoforms, mention has been made of "new genetic variants of A1BG."[39]
"Proteomic analysis revealed that [a circulating] set of plasma proteins was α 1 B-glycoprotein (A1BG) and its post-translationally modified isoforms."[40]
Pharmacogenomic variants have been reported. There are A1BG genotypes.[41]
A1BG has a genetic risk score of rs893184.[41]
"A genetic risk score, including rs16982743, rs893184, and rs4525 in F5, was significantly associated with treatment-related adverse cardiovascular outcomes in whites and Hispanics from the INVEST study and in the Nordic Diltiazem study (meta-analysis interaction P=2.39×10−5)."[41]
"rs893184 causes a histidine (His) to arginine (Arg) [nonsynonymous single nucleotide polymorphism (nsSNP), A (minor) for G (major)] substitution at amino acid position 52 in A1BG."[41]
For example, GeneID: 9 has isoforms: a, b, X1, and X2. Each of these (a and b) have variants. Variants 1-6 and 9 all encode the same isoform (a).
Variants 7, 8 and 10 all encode isoform b. Isoforms X1 and X2 are predicted.
Variants can differ in promoters, untranslated regions, or exons. For GeneID: 9: This variant (1) represents the longest transcript but encodes the shorter isoform (a). This variant is transcribed from a promoter known as P1, promoter 2, or NATb promoter.
This variant (2, also known as Type IID) lacks an alternate exon in the 5' UTR, compared to variant 1. This variant is transcribed from a promoter known as P1, promoter 2, or NATb promoter.
This variant (9, also known as Type IA) has a distinct 5' UTR and represents use of an alternate promoter known as the NATa or P3 promoter, compared to variant 1.
But, A1BG in NCBI Gene lists only one isoform, the gene locus itself, and the protein transcribed is a precursor subject to translational or more likely post-translational modifications.
The presence of multiple MREs coupled with experimental results from the literature indicating post-translational isoforms tends to confirm the existence of two or more isoforms for A1BG.
It isn't known which, if any, assist in locating and affixing the transcription mechanism for A1BG. This examination is the first to test one such DNA-occurring TF: the HNF6s.
The presence of multiple MREs coupled with experimental results from the literature indicating post-translational isoforms tends to confirm the existence of two or more isoforms for A1BG and likely transcription from either side.
Motif ten elements
No Motif ten elements occur on either side of A1BG.
STAT5s
STAT5s have a role as downstream signal transducers in A1BG, where the murine downstream promoter element is only 11 nts displaced from the human one. This suggests a STAT5 participation in human gene transcription of A1BG in the proximal promoter downstream between any other promoter and the TSS on the ZNF497 side of A1BG.
A1BG is not transcribed by any STAT5s is clearly disproved by the STAT5 transcription factor in the proximal promoter on the ZNF497 side of A1BG.
STAT5s may assist transcription of A1BG by other transcription factors, literature search has found that STAT5s assist transcription of A1BG by other transcription factors.[37] The proximal STAT5 promoter is -58 to -50 from A1BG TSS. If another STAT5 promoter is at -2.3 kb, it is about -1.4 kb inside ZNF497 which is 3212 nts long. Per analogy to the rat this would be expected.[37] A STAT5 transcription site lies at 3'-TTCCGGGAA-5' at 4247 in the proximal promoter, i.e. from 4242 (-58) to 4250 (-50). This suggests that STAT5 assists in the transcription of A1BG.
TATA boxes
On the negative strand in the negative direction (from ZSCAN22 to A1BG), looking for 3'-TATA-A/T-A-A/T-A/G-5', there no TATA boxes in the core promoter.
On the negative strand in the positive direction (from ZNF497 to A1BG), looking for 3'-TATA-A/T-A-A/T-A/G-5-5', there no TATA boxes in the core promoter.
There are no TATA boxes in the negative direction of the proximal promoter.
There are no TATA boxes in the positive direction of the proximal promoter.
For the positive strand in the negative direction looking for 3'-TATA-A/T-A-A/T-A/G-5', there's one 3'-TATATAAA-5' at 2874 nts, its complement and inverse complement of the distal promoter.
Any TATA boxes before A1BG from the direction of ZN497 may be out to 2300 nts. None were found in the distal promoter.
On the positive strand, in the nucleotide region between gene ZSCAN22 (NCBI GeneID: 342945) and A1BG (NCBI GeneID: 1) are 211 TATA box-like 8 nt long sequences. Of these,
- TATAAAAG occurs at 58853713 + 183 nts and
- TATAAAAG at 58853713 + 222. This is a TATA box found with some genes.[42] But, the optimal TBP recognition sequence 3'-TATATAAG-5',[43] does not occur.
- TATATAAA occurs only once at 2874 nts from the end of ZSCAN22. TBP is bound to this sequence and TATAAAAG above.[44][45]
- TATAAA occurs seven times, with the closest one at 2874 nts from the end of ZSCAN22. "In virtually every RNA polymerase II-transcribed gene examined, the sequence TATAAA was present 25 to 30 nts upstream of the transcription start site."[17]
A1BG does not have a TATA box in the core promoter region. There is the sequence 3'-TGCTATATAGATGGCAACTAAGCACTTGGGGAAAAAA-5' for which the first nt (T) is number 58856598 or 1574 nt upstream from the beginning of the 3'-UTR at 58858172. Unless another variant exists, -1574 nt from the beginning of the 3'-UTR is a large number of nts away from the TSS.
The closest TATA box-like sequence is 3'-CTCTTAAG-5' on the template strand at 4408 nts from the end of ZSCAN22, which is upstream from the core promoter.
The extra TATA boxes between ZSCAN22 and A1BG strongly suggest that there is at least one gene (or pseudogene) between ZSCAN22 and A1BG not currently in the NCBI database.
On the negative strand between ZNF497 and A1BG, there are no TATA boxes of the form 3’-TATA-A/T-A-A/T-A/G-5’.
For the negative strand going from ZSCAN22 to A1BG there are two TATA boxes: 3'-TATATATA-5' at 1600 nts and 3'-TATATAAA-5' at 1602 nts. These are way too far from the possible TSS in this direction.
These two TATA boxes in the distal promoter at approximately -2860 nts from the TSS suggest that there may be a short gene between ZSCAN22 and A1BG.
The hypothesis: TATA boxes are not involved in the transcription of A1BG is true. "In virtually every RNA polymerase II-transcribed gene examined, the sequence TATAAA was present 25 to 30 nts upstream of the transcription start site."[17]
There are no TATA boxes at all between ZNF497 and A1BG.
On the negative strand between ZSCAN22 and A1BG there are many TATA boxes between 184 nts from ZSCAN22 and 2874 nts from ZSCAN22 yet no genes are apparently known to occur between ZSCAN22 and A1BG. ZSCAN22 has several isoforms but all end exactly at the one TSS on the A1BG side.
From the number and variety of TFs on both sides of A1BG, multiple transcriptions should be possible. Any connection between bone, muscle and brain mass loss and A1BG likely uses one or more of the sides, directions, or forms (16 ways) and includes one or more TFs. Determining which produces deleterious effects is the first step toward reversal in a zero-g radiation inducing environment.
TATCCAC boxes
No TATCCAC boxes occur on either side of A1BG.
X boxes
No X boxes occur on either side of A1BG.
Y boxes
No Y boxes occur on either side of A1BG.
Experiments
The Basic programs (starting with SuccessablesGARC.bas) were written to compare nucleotide sequences with the sequences on either the template strand (-), or coding strand (+), of the DNA, in the negative direction (-), or the positive direction (+), including the extended number of nts from 958 to 4445, looking for GA responsive complex elements or boxes, their possible complements and inverses, to test the hypothesis that GARC boxes are not involved in the transcription of A1BG.
Literature searches were performed to determine the likely TFs that are members of the GA responsive complexes and with A1BG for possible interactions.
Results
Members of the GARC were found to include
- the TAACAAA box or GA responsive element (GARE),[1]
- the pyrimidine box CCTTTT,[1]
- the TATCCAC box,[1]
- the W boxes,[3]
- GARE-like elements (TAACAA/GA, or TAACGTA),[3]
- D-3 (AAAAG),[2]
- TAT boxes (TATCCAT),[3]
- CARE (CAACTC) regulatory elements,[3] and/or
- the TATC boxes.[5]
The TATC boxes are the same as the TATCCAC boxes.[15] D-3 (AAAAG) is a inverse complement of a portion of the pyrimidine box.
GAREs
For the GARE, the programmed sampling found no GARE in the core promoters on either side of A1BG.
No GARE occur within the proximal promoter on either side of A1BG.
But, an inverse GARE: 3'-AAACAAT-5' and its complement at 230 nts occur close to ZSCAN22, likely way outside the distal promoters, so no GARE occur on either side of A1BG in the distal promoters.
Pyrimidine boxes
No Pyrimidine boxes occur in the core promoters on either side of A1BG.
No Pyrimidine boxes occur within the proximal promoter on either side of A1BG.
Pyrimidine boxes and their complements: 3'-CCTTTT-5' at 2459, 3'-CCTTTT-5' at 2927, and 3'-CCTTTT-5' at 2968 occur in the negative direction. Inverse pyrimidine boxes and their complements occur 3'-AAAAGG-5' at 105, 3'-AAAAGG-5' at 1107, 3'-AAAAGG-5' at 3345, and 3'-AAAAGG-5' at 3441 also in the negative direction.
A pyrimidine box in the positive direction 3'-CCTTTT-5' at 135 and 3'-CCTTTT-5' at 291 and their complements occur close to ZNF497.
TATCCAC boxes
No TATCCAC boxes occur in the core promoters on either side of A1BG.
No TATCCAC boxes occur within the proximal promoter on either side of A1BG.
No TATCCAC boxes occur on either side of A1BG in the distal promoters.
W boxes
No W boxes occur in the core promoters on either side of A1BG.
But, inverse W boxes occur within the proximal promoter in the negative direction of A1BG: 3'-GGTCAA-5' at 4416 and 3'-GGTCAA-5' at 4308.
W boxes occur within the proximal promoter in the positive direction of A1BG: 3'-CTGACC-5' and its complement at 4216 and inverse W boxes occur 3'-GGTCAG-5' and its complement at 4270.
A W box occurs 3'-CTGACC-5' at 3749, whereas 3'-CTGACT-5' at 17, 3'-TTGACT-5' at 130, 3'-TTGACT-5' at 307, and 3'-CTGACC-5' at 734 occur close to ZSCAN22, but 3'-CTGACT-5' at 1935 could be associated ZSCAN22 or an unknown gene between it and A1BG, along with their complements in the negative direction of the distal promoter.
W box inverses occur 3'-GGTCAG-5' at 1353 and 3'-AGTCAG-5' at 2101, 3'-GGTCAG-5' at 2221, 3'-AGTCAG-5' at 2608, 3'-AGTCAA-5' at 2614, and 3'-AGTCAG-5' at 2619 along with their complements.
W boxes occur 3'-CTGACC-5' at 1662, 3'-CTGACC-5' at 2213, 3'-TTGACC-5' at 2873, 3'-CTGACT-5' at 2945, and 3'-TTGACC-5' at 4018 that could be associated with A1BG, along with 3'-TTGACC-5' at 1953, 3'-CTGACT-5' at 2674, and 3'-TTGACT-5' at 3735 in the positive direction of the distal promoter.
TAT boxes
No TAT boxes occur in the core promoters on either side of A1BG.
No TAT boxes occur within the proximal promoter on either side of A1BG.
An inverse TAT box occurs 3'-TACCTAT-5' at 2996 with its complement in the negative direction of the distal promoter.
No TAT boxes occur in the positive direction of the distal promoter.
CAREs
No CARE occur in the core promoters on either side of A1BG.
No CARE occur within the proximal promoter on either side of A1BG.
A CARE occurs 3'-CAACTC-5' at 86 possibly associated with ZSCAN22. But inverse CARE occur 3'-CTCAAC-5' at 1406, 3'-CTCAAC-5' at 2592, 3'-CTCAAC-5' at 2704, 3'-CTCAAC-5' at 3115, and 3'-CTCAAC-5' at 4096 in the negative direction of the distal promoter.
A CARE occurs 3'-CAACTC-5' at 3292 in the positive direction. But inverse CARE occur 3'-CTCAAC-5' at 1406 and 3'-CTCAAC-5' at 1621 and 3'-CTCAAC-5' at 3290, in the positive direction of the distal promoter.
Discussions
As found earlier the TATCCAC box is not present in any promoter for A1BG so it is not used to transcribe A1BG. But, can the GA responsive complex function transcriptionally without the TATCCAC or TATC box?
TATC boxes
With the TATC box (GARE), (pyrimidine box):
- "Upon cereal seed germination, the seed storage proteins (SSP) and defence proteins (CMe) whose mRNAs are abundant during the maturation phase are silenced. Instead, a different set of genes, encoding hydrolases, is induced in the aleurone upon GA perception. The promoter regions of these genes, such as those encoding α-amylases and proteases (Cejudo et al., 1992; Cercos et al., 1999; Gubler et al., 1995, 1999; Mena et al., 2002), contain the conserved GA response complex (GARC), a tripartite cis motif comprising the GA response element (GARE, 5'-TAACAAA-3'), the pyrimidine box (5'-CTTTT-3') and the TATC box (5'-TATCCAC-3'), all of which are necessary for a full GA response."[15]
- "To identify the reason of the low-level expression of BnGID1 in mutant, we sequenced the upstream sequence of BnGID1 and found that there was a GA-responsive complex including GARE (MBS), pyrimidine box and TATC-box, which is same as the upstream sequence of AtGID1a."[5]
- "Although this GARC [GA responsive complex] may not always be tripartite, most often it includes three sequence motifs, the TAACAAA box or GA responsive element (GARE), the pyrimidine box CCTTTT, and the TATCCAC box (Skriver et al., 1991;Gubler and Jacobsen, 1992; Rogers et al., 1994)."[1]
Without the TATC box (pyrimidine box), (TAT box), (GARE), (CARE), and (W box):
- "Some other cis-acting elements, such as pyrimidine boxes (GGTTTT) and TAT boxes (TATCCAT), are usually present in the vicinity of the GARE sequence of genes regulated by GA in cereal aleurone cells (Gubler and Jacobsen 1992; Cercos et al. 1999; Tsuji et al. 2006). For example, GARE and a novel CARE (CAACTC regulatory elements) elements are present in the promoter of rice RAmy1A (Ueguchi-Tanaka et al. 2000; Sutoh and Yamauchi 2003). Cis-element analyses have shown that the OsGAMYB protein activates RAmy1A expression through interaction with GARE in the promoter (Washio 2003). In addition, GARE and CARE are also present in a cysteine proteinase gene REP-1, which is expressed in rice aleurone and is induced by GAs and repressed by ABA. These two elements have been identified as necessary and sufficient for conferring GA inducibility of the REP-1 promoter. Mutations of CARE in the promoters of RAmy1A and REP-1 result in loss of GA inducibility and GAMYB transactivation, suggesting that CARE is a regulatory element for the GA-inducible expression of hydrolase genes in germinating seeds (Sutoh and Yamauchi 2003)."[3]
- "The pyrimidine box is another promoter element that is observed in cereal GA-responsive promoters examined thus far (Huang et al., 1990). Mutations on the pyrimidine box caused the reduction of the GA induction with a lesser magnitude than those of GARE in the GA-treated aleurone (Gubler and Jacobsen, 1992; Rogers and Rogers, 1992). The pyrimidine box alone could not confer the hormone responsiveness to the minimal promoter of the cauliflower mosaic virus 35S (CaMV35S), suggesting accessory roles of the pyrimidine box on the transcription response to GA (Skriver et al., 1991)."[2]
- The "presence of WRKY TF binding sites (C/TTGACC/T, W boxes) in numerous co-regulated Arabidopsis defense gene promoters provided circumstantial evidence that zinc-finger-type WRKY factors play a broad and pivotal role in regulating defenses [10]."[6]
Zinc fingers
Zinc fingers occur on both sides of A1BG: ZSCAN22 (GeneID: 342945) zinc finger and SCAN domain containing 22 and ZNF497 (Gene ID: 162968) zinc finger protein 497.
"Sequence analysis shows that the OsDof3 cDNA encodes a 371-amino acid polypeptide related to the PBF factors of cereal plants. The predicted amino acid sequence of OsDOF3 aligns well with those of maize (Zea mays; Vicente-Carbajosa et al., 1997), wheat (Triticum aestivum), and barley PBF proteins (Mena et al., 1998; [...]). The N-terminal sequence of OsDOF3 contains four Cys residues reminiscent of the Dof zinc finger and shows around 80% sequence identities with PBFs, whereas the C-terminal parts are divergent showing several insertions and deletions."[2]
"Pentanucleotide sequence from the pyrimidine box (CTTTT; Huang et al., 1990) and D-3 (AAAAG) is capable of matching a favored substrate selected by in vitro DNA binding of maize Dof proteins (CTTTT or AAAAG; Yanagisawa and Schmidt, 1999). These results indicate preferential binding of OsDOF3 to two pyrimidine box and D-3 motifs in the RAmy1A promoter context."[2]
The pyrimidine boxes are likely associated with zinc fingers.
An AGC box was found in the distal promoter of either gene ZSCAN22 or A1BG on both the template and coding strands.
A full web search produced several references including a GeneCard[26] for "zinc finger protein 497" and "GCC box", including "May be involved in transcriptional regulation."[26] Zinc fingers are mentioned in association with GCC boxes in plants.
An extension of the nucleotide data for the positive direction from ZNF475 toward A1BG from 958 nts to 4445 nts has not discovered any AGC boxes even in the distal promoter just beyond ZNF497.
"The human genome is estimated to contain 700 zinc-finger genes, which perform many key functions, including regulating transcription. [Four] clusters of zinc-finger genes [occur] on human chromosome 19".[38]
Nearby zinc-fingers on chromosome 19 include ZNF497 (GeneID: 162968), ZNF837 (GeneID: 116412), and ZNF8 (GeneID: 7554).
"In rodents and in humans, about one third of the zinc-finger genes carry the Krüppel-associated box (KRAB), a potent repressor of transcription (Margolin et al. 1994), [...]. There are more than 200 KRAB-containing zinc-finger genes in the human genome, about 40% of which reside on chromosome 19 and show a clustered organization suggesting an evolutionary history of duplication events (Dehal et al. 2001)."[38]
ZNF8 is in cluster V along with A1BG.[38]
"In contrast to the four clusters considered [I through IV], one that occurs at the telomere of chromosome 19, which we will call cluster V, has been very stable [over mouse, rat, and human]."[38]
"Apart from the somewhat unexpected location of Zfp35 on mouse chromosome 18 and of the AIBG orthologs on mouse chromosome 15 and rat chromosome 7, there has been little rearrangement."[38]
So far no article has reported any linkage between zinc, including various zinc fingers, or cadmium, and A1BG.
GAREs
No GARE occur on either side of A1BG in the distal promoters.
Can the GA responsive complex function transcriptionally without the GARE?
No (pyrimidine box), (TAT box), (CARE):
- "The pyrimidine box alone could not confer the hormone responsiveness to the minimal promoter of the cauliflower mosaic virus 35S (CaMV35S), suggesting accessory roles of the pyrimidine box on the transcription response to GA (Skriver et al., 1991)."[2]
- "Some other cis-acting elements, such as pyrimidine boxes (GGTTTT) and TAT boxes (TATCCAT), are usually present in the vicinity of the GARE sequence of genes regulated by GA in cereal aleurone cells (Gubler and Jacobsen 1992; Cercos et al. 1999; Tsuji et al. 2006). For example, GARE and a novel CARE (CAACTC regulatory elements) elements are present in the promoter of rice RAmy1A (Ueguchi-Tanaka et al. 2000; Sutoh and Yamauchi 2003). Cis-element analyses have shown that the OsGAMYB protein activates RAmy1A expression through interaction with GARE in the promoter (Washio 2003). In addition, GARE and CARE are also present in a cysteine proteinase gene REP-1, which is expressed in rice aleurone and is induced by GAs and repressed by ABA. These two elements have been identified as necessary and sufficient for conferring GA inducibility of the REP-1 promoter. Mutations of CARE in the promoters of RAmy1A and REP-1 result in loss of GA inducibility and GAMYB transactivation, suggesting that CARE is a regulatory element for the GA-inducible expression of hydrolase genes in germinating seeds (Sutoh and Yamauchi 2003)."[3]
- "Given the general observations that a couple of the promoter motifs, GARE and the pyrimidine box, always exist in a close distance in the reported GA-responsive promoters (Huang et al., 1990), the synergistic effect on RAmy1A promoter activation are likely to be mediated through protein-protein interaction."[2]
Yes (W box):
- "Plant immune responses are associated with the concerted modulation of a large number of different WRKY transcripts and proteins [15,34–36,37**]. Upon triggering of SA-dependent defenses, at least 49 AtWRKY genes exhibited differential regulation representing separate waves of transcript accumulation or repression [34]. Their promoters are statistically enriched for W boxes, suggesting that they are autoregulated or controlled by other WRKY proteins [34]."[6]
W boxes for A1BG
W boxes occur in some form in the proximal and distal promoters on both sides of A1BG. As such A1BG could be transcribed by W boxes acting alone or in concert with other TFs.
Conclusions
Regarding the research hypothesis: GA responsive complexes are not involved in the transcription of A1BG. This seems unlikely. While a GA responsive complex does not occur on either side of A1BG, the GA responsive complex element, W boxes, do and may be involved in the transcription of A1BG, or could be involved if appropriately stimulated.
Laboratory evaluations
To assess your example, including your justification, analysis and discussion, I will provide such an assessment of my example for comparison and consideration.
Evaluation
No wet chemistry experiments were performed to confirm that Gene ID: 1 may be transcribed from either side using TFs in proximal promoters. The NCBI database is generalized, whereas individual human genome testing could demonstrate that A1BG is transcribed from either side TFs. Sufficient nts have been added to the data sets for the ZNF497 side to confirm likely transcription of A1BG.
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Montaña Mena, Francisco Javier Cejudo, Ines Isabel-Lamoneda and Pilar Carbonero (1 September 2002). "A Role for the DOF Transcription Factor BPBF in the Regulation of Gibberellin-Responsive Genes in Barley Aleurone". Plant Physiology. 130 (1): 111–9. doi:10.1104/pp.005561. Retrieved 2017-02-19.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 Kenji Washio (October 2003). "Functional Dissections between GAMYB and Dof Transcription Factors Suggest a Role for Protein-Protein Associations in the Gibberellin-Mediated Expression of the RAmy1A Gene in the Rice Aleurone" (PDF). Plant Physiology. 133 (2): 850–63. doi:10.1104/pp.103.027334. Retrieved 10 October 2018.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Liu-Min Fan, Xiaoyan Feng, Yu Wang and Xing Wang Deng (2007). "Gibberellin Signal Transduction in Rice". Journal of Integrative Plant Biology. 49 (6): 731−741. doi:10.1111/j.1744-7909.2007.00511.x. Retrieved 16 October 2018.
- ↑ Fiona J. Woodger, Frank Gubler, Barry J. Pogson and John V. Jacobsen (2003). "A Mak-like kinase is a repressor of GAMYB in barley aleurone". The Plant Journal. 33: 707–717. doi:10.1046/j.1365-313X.2003.01663.x. Retrieved 16 October 2018.
- ↑ 5.0 5.1 5.2 Huapeng Li, Yun Wang, Xiaocheng Li, Yong Gao, Zhijun Wang, Yun Zhao and Maolin Wang (January 2011). "A GA-insensitive dwarf mutant of Brassica napus L. correlated with mutation in pyrimidine box in the promoter of GID1". Molecular Biology Reports. 38 (1): 191–197. doi:10.1007/s11033-010-0094-2. Retrieved 10 October 2018.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Thomas Eulgem and Imre E Somssich (2007). "Networks of WRKY transcription factors in defense signaling" (PDF). Current Opinion in Plant Biology. 10: 366–371. doi:10.1016/j.pbi.2007.04.020. Retrieved 17 October 2018.
- ↑ Rushton, Paul; Macdonald, H.; Huttly, A.K.; Lazarus, C.M.; Hooley, R (1995). "Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of alpha-Amy2 genes". Plant Molecular Biology. 29: 691–702. doi:10.1007/bf00041160. PMID 8541496.
- ↑ 8.0 8.1 Rushton PJ, Somssich IE, Ringler P, Shen QJ (May 2010). "WRKY transcription factors". Trends Plant Science. 15 (5): 247–58. doi:10.1016/j.tplants.2010.02.006. PMID 20304701.
- ↑ 9.0 9.1 Rushton, Paul. "The Lab of Dr. Paul Rushton". wordpress.com. Retrieved 17 June 2013.
- ↑ Ciolkowski, I.; Wanke D; Birkenbihl RP; Somssich IE. (2008). "Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function". Plant Mol Biol. 68: 81–92. doi:10.1007/s11103-008-9353-1. PMC 2493524. PMID 18523729.
- ↑ 11.0 11.1 Brand; Fischer; Harter; Kohlbacher; Wanke (2013). "Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays". Nucleic Acids Research. 41 (21): 9764–9778. doi:10.1093/nar/gkt732.
- ↑ 12.0 12.1 Yamasaki, K.; Kigawa T; Watanabe S; Inoue M; Yamasaki T; Seki M; Shinozaki K; Yokoyama S. (2012). "Structural basis for sequence-specific DNA recognition by an Arabidopsis WRKY transcription factor". J. Biol. Chem. 287: 7683–91. doi:10.1074/jbc.M111.279844. PMC 3293589. PMID 22219184.
- ↑ Zu‐Jun Yang, Guang‐Rong Li, Huan‐Lin Shu, Chang Liu, Juan Feng, Zhi‐Jian Chang and Zheng‐Long Ren (31 August 2006). "Molecular characterization of high molecular weight glutenin subunit allele 1Bx23 in common wheat introduced from hexaploid triticale". Hereditas. 143 (December): 159–166. doi:10.1111/j.2006.0018-0661.01948.x. Retrieved 17 October 2018.
- ↑ Ben C. Berks, Tracy Palmer and Frank Sargent (2004). "The Tat protein translocation pathway and its role in microbial physiology" (PDF). Advances in Microbial Physiology. 47: 187–254. doi:10.1016/S0065-2911(03)47004-5. Retrieved 17 October 2018.
- ↑ 15.0 15.1 15.2 I. Rubio-Somoza, M. Martinez, Z. Abraham, I. Diaz and P. Carbonero (July 2006). "Ternary complex formation between HvMYBS3 and other factors involved in transcriptional control in barley seeds". The Plant Journal. 47 (2): 269–281. doi:10.1111/j.1365-313X.2006.02777.x. Retrieved 17 October 2018.
- ↑ Jennifer E.F. Butler, James T. Kadonaga (October 15, 2002). "The RNA polymerase II core promoter: a key component in the regulation of gene expression". Genes & Development. 16 (20): 2583–292. doi:10.1101/gad.1026202. PMID 12381658.
- ↑ 17.0 17.1 17.2 Stephen T. Smale and James T. Kadonaga (July 2003). "The RNA Polymerase II Core Promoter" (PDF). Annual Review of Biochemistry. 72 (1): 449–79. doi:10.1146/annurev.biochem.72.121801.161520. PMID 12651739. Retrieved 2012-05-07.
- ↑ Thomas Shafee and Rohan Lowe (9 March 2017). "Eukaryotic and prokaryotic gene structure" (PDF). WikiJournal of Medicine. 4 (1): 2. doi:10.15347/wjm/2017.002. Retrieved 2017-04-06.
- ↑ Koichi Takayama, Ken-ichirou Morohashi, Shin-ichlro Honda, Nobuyuki Hara and Tsuneo Omura (1 July 1994). "Contribution of Ad4BP, a Steroidogenic Cell-Specific Transcription Factor, to Regulation of the Human CYP11A and Bovine CYP11B Genes through Their Distal Promoters". The Journal of Biochemistry. 116 (1): 193–203. doi:10.1093/oxfordjournals.jbchem.a124493. Retrieved 2017-08-16.
- ↑ Michelle Craig Barton, Navid Madani, and Beverly M. Emerson (8 July 1997). "Distal enhancer regulation by promoter derepression in topologically constrained DNA in vitro". Proceedings of the National Academy of Sciences of the United States of America. 94 (14): 7257–62. Retrieved 2017-08-16.
- ↑ A Aoyama, T Tamura, K Mikoshiba (March 1990). "Regulation of brain-specific transcription of the mouse myelin basic protein gene: function of the NFI-binding site in the distal promoter". Biochemical and Biophysical Research Communications. 167 (2): 648–53. doi:10.1016/0006-291X(90)92074-A. Retrieved 2012-12-13.
- ↑ J Gao and L Tseng (June 1996). "Distal Sp3 binding sites in the hIGBP-1 gene promoter suppress transcriptional repression in decidualized human endometrial stromal cells: identification of a novel Sp3 form in decidual cells". Molecular Endocrinology. 10 (6): 613–21. doi:10.1210/me.10.6.613. Retrieved 2012-12-13.
- ↑ Peter Pasceri, Dylan Pannell, Xiumei Wu, and James Ellis (July 15, 1998). "Full activity from human β-globin locus control region transgenes requires 5′ HS1, distal β-globin promoter, and 3′ β-globin sequences". Blood. 92 (2): 653–63. Retrieved 2012-12-13.
- ↑ Virginia González-Calle, Raquel Iglesias-Fernández, Pilar Carbonero, and Cristina Barrero-Sicilia (September 2014). "The BdGAMYB protein from Brachypodium distachyon interacts with BdDOF24 and regulates transcription of the BdCathB gene upon seed germination" (PDF). Planta. 240 (3): 539–552. doi:10.1007/s00425-014-2105-3. Retrieved 16 October 2018.
- ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 Marcelo S da Silva, Arina M Perez, Rita de Cássia V da Silveira, Camila E de Moraes, Jair L Siqueira-Neto, Lucio H Freitas-Junior & Maria Isabel N Cano (7 May 2010). "The Leishmania amazonensis TRF (TTAGGG repeat-binding factor) homologue binds and co-localizes with telomeres". BMC Microbiology. 10: 136. Retrieved 16 January 2020.
- ↑ 26.0 26.1 26.2 26.3 Weizmann Institute of Science (2017). Zinc Finger Protein 497. Israel: Weizmann Institute of Science. Retrieved 2017-08-20.
- ↑ Annie Charbonneau and Van-Luu The (26 January 2001). "Genomic organization of a human 5β-reductase and its pseudogene and substrate selectivity of the expressed enzyme". Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 1517 (2): 228–235. doi:10.1016/S0167-4781(00)00278-5. Retrieved 2017-11-17.
- ↑ RefSeq (12 May 2018). GAS5 growth arrest specific 5 (non-protein coding) [ Homo sapiens (human) ]. 8600 Rockville Pike, Bethesda MD, 20894 USA: National Center for Biotechnology Information, U.S. National Library of Medicine. Retrieved 12 May 2018.
- ↑ Satu Nahkuri, Ryan J. Taft, Darren J. Korbie and John S. Mattick (12 September 2008). "Molecular Evolution of the HBII-52 snoRNA Cluster". Journal of Molecular Biology. 381 (4): 810–815. doi:10.1016/j.jmb.2008.06.057. Retrieved 2018-05-16.
- ↑ Kevin Roy and Guillaume F. Chanfreau (2012). Eukaryotic RNases and their Partners in RNA Degradation and Biogenesis, Part A, In: The Enzymes. sciencedirect. Retrieved 16 May 2018.
- ↑ Yannick Delpu, Dorian Larrieu, Marion Gayral, Dina Arvanitis, Marlène Dufresne, Pierre Cordelier, Jérôme Torrisani (2016). Gerda Egger and Paola Arimondo, ed. Noncoding RNAs, In: Drug Discovery in Cancer Epigenetics. sciencedirect. pp. 305–326. ISBN 978-0-12-802208-5. Retrieved 16 May 2018.
- ↑ RJ Taft, EA Glazov, N Cloonan, C Simons, S Stephen, GJ Faulkner, T, Lassmann, AR Forrest, SM Grimmond, K Schroder, K Irvine, T Arakawa, M Nakamura, A Kubosaki, K Hayashida, C Kawazu, M Murata, H Nishiyori, S Fukuda, J Kawai, CO Daub, DA Hume, H Suzuki, V Orlando, P Carninci, Y Hayashizaki, JS Mattick (May 2009). "Tiny RNAs associated with transcription start sites in animals". Nature Genetcs. 41 (5): 572–8. doi:10.1038/ng.312. Retrieved 2018-05-16.
- ↑ 33.0 33.1 Markus Brameier, Astrid Herwig, Richard Reinhardt, Lutz Walter, Jens Gruber (1 January 2011). "Human box C/D snoRNAs with miRNA like functions: expanding the range of regulatory RNAs". Nucleic Acids Research. 39 (2): 675–686. doi:10.1093/nar/gkq776. Retrieved 2018-05-16.
- ↑ RefSeqNov2015 (November 2015). VEGFA vascular endothelial growth factor A [Homo sapiens (human)]. 8600 Rockville Pike, Bethesda 0894 USA: National Center for Biotechnology Information, U.S. National Library of Medicine. Retrieved 22 May 2018.
- ↑ Weiwei Deng, Hua Ying, Chris A. Helliwell, Jennifer M. Taylor, W. James Peacock, and Elizabeth S. Dennis (19 April 2011). "FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis". Proceedings of the National Academy of Sciences United States of America. 108 (16): 6680–6685. doi:10.1073/pnas.1103175108. Retrieved 2017-09-17.
- ↑ Thomas K Albert, Korbinian Grote, Stefan Boeing and Michael Meisterernst (15 March 2010). "Basal core promoters control the equilibrium between negative cofactor 2 and preinitiation complexes in human cells". Genome Biology. 11: R33. doi:10.1186/gb-2010-11-3-r33.
|access-date=requires|url=(help) - ↑ 37.00 37.01 37.02 37.03 37.04 37.05 37.06 37.07 37.08 37.09 37.10 37.11 37.12 37.13 37.14 37.15 Cissi Gardmo and Agneta Mode (1 December 2006). "In vivo transfection of rat liver discloses binding sites conveying GH-dependent and female-specific gene expression". Journal of Molecular Endocrinology. 37 (3): 433–441. doi:10.1677/jme.1.02116. Retrieved 2017-09-01.
- ↑ 38.0 38.1 38.2 38.3 38.4 38.5 38.6 38.7 38.8 38.9 Deena Schmidt and Rick Durrett (1 December 2004). "Adaptive Evolution Drives the Diversification of Zinc-Finger Binding Domains". Molecular Biology and Evolution. 21 (12): 2326–2339. doi:10.1093/molbev/msh246. Retrieved 2017-10-16.
- ↑ H Eiberg, ML Bisgaard, J Mohr (1 December 1989). "Linkage between alpha 1B-glycoprotein (A1BG) and Lutheran (LU) red blood group system: assignment to chromosome 19: new genetic variants of A1BG". Clinical genetics. 36 (6): 415–8. PMID 2591067. Retrieved 2017-10-08.
- ↑ John R. Stehle Jr., Mark E. Weeks, Kai Lin, Mark C. Willingham, Amy M. Hicks, John F. Timms, Zheng Cui (January 2007). "Mass spectrometry identification of circulating alpha-1-B glycoprotein, increased in aged female C57BL/6 mice". Biochimica et Biophysica Acta (BBA) - General Subjects. 1770 (1): 79–86. Retrieved 2017-10-08.
- ↑ 41.0 41.1 41.2 41.3 Caitrin W. McDonough, Yan Gong, Sandosh Padmanabhan, Ben Burkley, Taimour Y. Langaee, Olle Melander, Carl J. Pepine, Anna F. Dominiczak, Rhonda M. Cooper-DeHoff, Julie A. Johnson (June 2013). "Pharmacogenomic Association of Nonsynonymous SNPs in SIGLEC12, A1BG, and the Selectin Region and Cardiovascular Outcomes" (PDF). Hypertension. 62 (1): 48–54. doi:10.1161/HYPERTENSIONAHA.111.00823. PMID 23690342. Retrieved 2017-10-08.
- ↑ Georgia A. Patikoglou, Joseph L. Kim, Liping Sun, Sang-Hwa Yang, Thomas Kodadek, and Stephen K. Burley (1999). "TATA element recognition by the TATA box-binding protein has been conserved throughout evolution". Genes & Development. 13: 3217–30. Retrieved 2012-06-12.
- ↑ Jie Min Wong and Erik Bateman (25 May 1994). "TBP-DNA interactions in the minor groove discriminate between A: T and T: A base pairs". Nucleic Acids Research. 22 (10): 1890–96. doi:10.1093/nar/22.10.1890. Retrieved 2012-06-12.
- ↑ Joseph L. Kim, Dimitar B. Nikolov & Stephen K. Burley (7 October 1993). "Co-crystal structure of TBP recognizing the minor groove of a TATA element". Nature. 365: 520–7. doi:10.1038/365520a0. Retrieved 2012-06-12.
- ↑ Youngchang Kim, James. H. Geiger, Steven Hahn & Paul B. Sigler (7 October 1993). "Crystal structure of a yeast TBP/TATA-box complex". Nature. 365: 512–20. Retrieved 2012-06-12.