Chlamydophila trachomatis
| Chlamydia trachomatis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
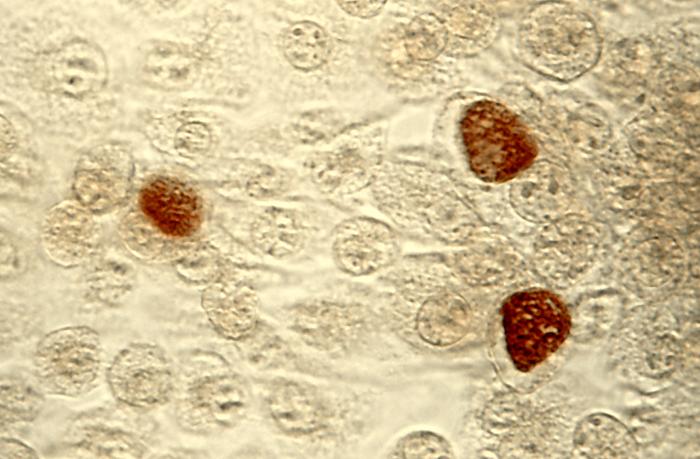 C. trachomatis inclusion bodies (brown) in a McCoy cell culture.
| ||||||||||
| Scientific classification | ||||||||||
| ||||||||||
| Species | ||||||||||
|
Chlamydia muridarum
Chlamydophila pneumoniae |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Chlamydia trachomatis, an obligate intracellular human pathogen, is one of four bacterial species in the genus Chlamydia.[1] C. trachomatis is a gram-negative bacterium, therefore its cell wall components retain the counter-stain safranin and appear pink under a light microscope.[2] It is ovoid in shape.[3] The inclusion bodies of Chlamydia trachomatis were first described in 1942; the Chlamydia trachomatis agent was first cultured in the yolk sacs of eggs by Professor Feifan Tang et al in 1957.[4][5]
C. trachomatis includes three human biovars:
- serovars Ab, B, Ba, or C — cause trachoma: infection of the eyes, which can lead to blindness
- serovars D-K — cause urethritis, pelvic inflammatory disease, ectopic pregnancy, neonatal pneumonia, and neonatal conjunctivitis
- serovars L1, L2 and L3 — lymphogranuloma venereum (LGV).[6]
Many, but not all, C. trachomatis strains have an extrachromosomal plasmid.[7]
Chlamydia can exchange DNA between its different strains, thus the evolution of new strains is common.[8]
Identification
Chlamydia species are readily identified and distinguished from other Chlamydia species using DNA-based tests.
Most strains of C. trachomatis are recognized by monoclonal antibodies (mAbs) to epitopes in the VS4 region of MOMP.[9] However, these mAbs may also cross-react with two other Chlamydia species, C. suis and C. muridarum.
Life-cycle
Clinical significance
Clinical signs and symptoms of C. trachomatis infection and Gonorrhea infection are indistinguishable.[10] C. trachomatis is the single most important infectious agent associated with blindness (trachoma); approximately 84 million worldwide suffer C. trachomatis eye infections and 8 million are blinded as a result of the infection.[11]
Laboratory tests
- Nucleic acid amplification tests (NAAT). These tests find the genetic material (DNA) of Chlamydia bacteria. These tests are the most sensitive tests available, meaning that they are very accurate and that they are very unlikely to have false-negative test results. A polymerase chain reaction (PCR) test is an example of a nucleic acid amplification test. This test can also be done on a urine sample.
- Nucleic acid hybridization tests (DNA probe test). A probe test also finds Chlamydia DNA. A probe test is very accurate but is not as sensitive as nucleic acid amplification tests.
- Enzyme-linked immunosorbent assay (ELISA, EIA). This quick test finds substances (Chlamydia antigens) that trigger the immune system to fight Chlamydia infection.
- Direct fluorescent antibody test (DFA). This quick test also finds Chlamydia antigens.
- Chlamydia cell culture. A test in which the suspected chlamydia sample is grown in a vial of cells. The pathogen infects the cells and after a set incubation time (48 hours) the vials are stained and viewed on a fluorescent light microscope. Cell culture is more expensive and takes longer (two days) than the other tests. The culture must be grown in a laboratory.[12]
Medical Therapy
Antimicrobial regimen
- 1 Chlaymydial infections treatment[13]
- 1.1 Chlamydial Infections in Adolescents and Adults
- Preferred regimen (1): Doxycycline 100 mg PO bid for 7 days
- Preferred regimen (2): Azithromycin 1 g PO in a single dose
- Alternative regimen (1): Erythromycin base 500 mg PO qid for 7 days
- Alternative regimen (2): Erythromycin ethylsuccinate 800 mg PO qid for 7 days
- Alternative regimen (3): Levofloxacin 500 mg PO qd for 7 days
- Alternative regimen (4): Ofloxacin 300 mg PO bid for 7 days.
- Note: Patients should be instructed to refer their sex partners for evaluation, testing, and treatment if they had sexual contact with the patient during the 60 days preceding onset of the patient's symptoms or chlamydia diagnosis.
- 1.2 Chlamydial Infections in patients with HIV Infection
- Preferred regimen (1): Doxycycline 100 mg PO bid for 7 days
- Preferred regimen (2): Azithromycin 1 g PO in a single dose
- Preferred regimen (3): Azithromycin 1 g PO in a single dose
- Alternative regimen (1): Erythromycin base 500 mg PO qid for 7 days
- Alternative regimen (2): Erythromycin ethylsuccinate 800 mg PO qid for 7 days
- Alternative regimen (3): Levofloxacin 500 mg PO qd for 7 days
- Alternative regimen (4): Ofloxacin 300 mg PO bid for 7 days.
- 1.3 Pregancy
- Preferred regimen: Azithromycin 1 g PO in a single dose
- Alternative regimen (1): Amoxicillin 500 mg PO tid for 7 days
- Alternative regimen (2): Erythromycin base 500 mg PO qid for 7 days
- Alternative regimen (3): Erythromycin base 250 mg PO qid for 14 days
- Alternative regimen (4): Erythromycin ethylsuccinate 800 mg PO qid for 7 days
- Alternative regimen (5): Erythromycin ethylsuccinate 400 mg PO qid for 14 days
- Note:Doxycycline, Ofloxacin, and Levofloxacin are contraindicated in pregnant women
- 1.4 Management of sex partners
- Preferred regimen (1): Doxycycline 100 mg PO bid for 7 days
- Preferred regimen (2): Azithromycin 1 g PO in a single dose
- Alternative regimen (1): Erythromycin base 500 mg PO qid for 7 days
- Alternative regimen (2): Erythromycin ethylsuccinate 800 mg PO qid for 7 days
- Alternative regimen (3): Levofloxacin 500 mg PO qd for 7 days
- Alternative regimen (4): Ofloxacin 300 mg PO bid for 7 days.
- Note (1): Recent sex partners (i.e., persons having sexual contact with the infected patient within the 60 days preceding onset of symptoms or Chlamydia diagnosis) should be referred for evaluation, testing, and presumptive dual treatment.
- Note (2): If the patient’s last potential sexual exposure was >60 days before onset of symptoms or diagnosis, the most recent sex partner should be treated.
- Note (3): To avoid reinfection, sex partners should be instructed to abstain from unprotected sexual intercourse for 7 days after they and their sexual partner(s) have completed treatment and after resolution of symptoms, if present
- 2. Chlamydial infection among neonates
- 2.1 Ophthalmia Neonatorumcaused by C. trachomatis
- Preferred regimen: Erythromycin base or ethylsuccinate 50 mg/kg/ day PO qid for 14 days
- Alternative regimen: Azithromycin suspension 20 mg/kg /day PO qd for 3 days
- Note: The mothers of infants who have chlamydial infection and the sex partners of these women should be evaluated and treated.
- 2.2 Infant Pneumonia
- Preferred regimen: Erythromycin base or ethylsuccinate 50 mg/kg/ day PO qid for 14 days
- Alternative regimen: Azithromycin suspension 20 mg/kg /day PO qd for 3 days
- 3.Chlamydial infection among infants and childern
- 3.1 Infants and childern who weigh < 45 kg
- Preferred regimen: Erythromycin base or ethylsuccinate 50 mg/kg/ day PO qid for 14 days
- 3.2 Infants and childern who weigh ≥45 kg but who are aged <8 years
- Preferred regimen: Azithromycin 1 g PO in a single dose
- 3.3 Infants and childern aged ≥8 years
- Preferred regimen (1): Azithromycin 1 g PO in a single dose
- Preferred regimen (2): Doxycycline 100 mg PO bid for 7 days
- 4. Lymphogranuloma venereum (LGV) [14]
- Preferred regimen: Doxycycline 100 mg PO bid for 21 days
- Alternative regimen: Erythromycin base 500 mg PO qid for 21 days
- Note (1): Azithromycin 1 g PO once weekly for 3 weeks is probably effective based on its chlamydial antimicrobial activity. Fluoroquinolone-based treatments might also be effective, but extended treatment intervals are likely required.
- Note (2): Pregnant and lactating women should be treated with Erythromycin. Azithromycin might prove useful for treatment of LGV in pregnancy, but no published data are available regarding its safety and efficacy. Doxycycline is contraindicated in pregnant women.
- Note (3): Persons with both LGV and HIV infection should receive the same regimens as those who are HIV negative. Prolonged therapy might be required, and delay in resolution of symptoms might occur.
- Note (4): Persons who have had sexual contact with a patient who has LGV within the 60 days before onset of the patient’s symptoms should be examined and tested for urethral, cervical, or rectal chlamydial infection depending on anatomic site of exposure. They should be presumptively treated with a chlamydia regimen ( Azithromycin 1 g PO single dose OR Doxycycline 100 mg PO bid for 7 days).
See also
References
- ↑ Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 463–70. ISBN 0-8385-8529-9.
- ↑ "Chlamydia". MicrobeWiki. Department of Biology, Kenyon College. 2006-08-15. Retrieved 2008-10-27.
- ↑ Malhotra M, Sood S, Mukherjee A, Muralidhar S, Bala M (September 2013). "Genital Chlamydia trachomatis: an update". Indian J. Med. Res. 138 (3): 303–16. PMC 3818592. PMID 24135174.
- ↑ Darougar S, Jones BR, Kinnison JR, Vaughan-Jackson JD, Dunlop EM (December 1972). "Chlamydial infection. Advances in the diagnostic isolation of Chlamydia, including TRIC agent, from the eye, genital tract, and rectum". Br J Vener Dis. 48 (6): 416–20. doi:10.1136/sti.48.6.416. PMC 1048360. PMID 4651177.
- ↑ Tang FF, Huang YT, Chang HL, Wong KC (1958). "Further studies on the isolation of the trachoma virus". Acta Virol. 2 (3): 164–70. PMID 13594716.
Tang FF, Chang HL, Huang YT, Wang KC (June 1957). "Studies on the etiology of trachoma with special reference to isolation of the virus in chick embryo". Chin Med J. 75 (6): 429–47. PMID 13461224.
Tang FF, Huang YT, Chang HL, Wong KC (1957). "Isolation of trachoma virus in chick embryo". J Hyg Epidemiol Microbiol Immunol. 1 (2): 109–20. PMID 13502539. - ↑ Fredlund H, Falk L, Jurstrand M, Unemo M (2004). "Molecular genetic methods for diagnosis and characterisation of Chlamydia trachomatis and Neisseria gonorrhoeae: impact on epidemiological surveillance and interventions". APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 112 (11–12): 771–84. doi:10.1111/j.1600-0463.2004.apm11211-1205.x. PMID 15638837.
- ↑ Carlson JH, Whitmire WM, Crane DD; et al. (June 2008). "The Chlamydia trachomatis Plasmid Is a Transcriptional Regulator of Chromosomal Genes and a Virulence Factor". Infection and immunity. 76 (6): 2273–83. doi:10.1128/IAI.00102-08. PMC 2423098. PMID 18347045.
- ↑ Harris SR, Clarke IN, Seth-Smith HM; et al. (April 2012). "Whole-genome analysis of diverse Chlamydia trachomatis strains identifies phylogenetic relationships masked by current clinical typing". Nat. Genet. 44 (4): 413–9, S1. doi:10.1038/ng.2214. PMC 3378690. PMID 22406642.
- ↑ Ortiz L, Angevine M, Kim SK, Watkins D, DeMars R (2000). "T-Cell Epitopes in Variable Segments of Chlamydia trachomatis Major Outer Membrane Protein Elicit Serovar-Specific Immune Responses in Infected Humans". Infect. Immun. 68 (3): 1719–23. doi:10.1128/IAI.68.3.1719-1723.2000. PMC 97337. PMID 10678996.
- ↑
- ↑ "Trachoma". Prevention of Blindness and Visual Impairment. World Health Organization.
- ↑ "Chlamydia Tests". Sexual Conditions Health Center. WebMD. Retrieved 2012-08-07.
- ↑ Workowski, Kimberly A.; Bolan, Gail A. (2015-06-05). "Sexually transmitted diseases treatment guidelines, 2015". MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 64 (RR-03): 1–137. ISSN 1545-8601. PMID 26042815.
- ↑ Workowski, Kimberly A.; Bolan, Gail A. (2015-06-05). "Sexually transmitted diseases treatment guidelines, 2015". MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 64 (RR-03): 1–137. ISSN 1545-8601. PMID 26042815.
Further reading
Bellaminutti, Serena; Seracini, Silva; De Seta, Francesco; Gheit, Tarik; Tommasino, Massimo; Comar, Manola (November 2014). "HPV and Chlamydia trachomatis Co-Detection in Young Asymptomatic Women from High Incidence Area for Cervical Cancer". Journal of Medical Virology. 86 (11): 1920–1925. doi:10.1002/jmv.24041. Retrieved 13 November 2014.
External links
- Chlamydiae.com
- Template:GPnotebook
- "Chlamydia trachomatis". NCBI Taxonomy Browser. 813.
Template:STD/STI Template:Gram-negative non-proteobacterial bacterial diseases[citation needed]
- Pages with reference errors
- CS1 maint: Extra text: authors list
- CS1 maint: Multiple names: authors list
- Pages with broken file links
- Chlamydiae
- Sexually transmitted diseases and infections
- Bacteria with sequenced genomes
- Infectious causes of cancer
- Infections with a predominantly sexual mode of transmission
- All articles with unsourced statements
- Articles with unsourced statements from August 2015
- Articles with invalid date parameter in template
- Eradicable diseases