Erdafitinib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Erdafitinib is a kinase inhibitor that is FDA approved for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma (mUC), that has susceptible FGFR3 or FGFR2 genetic alterations and progressed during or following at least one line of prior platinum-containing chemotherapy including within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy. Common adverse reactions include phosphate increased, stomatitis, fatigue, creatinine increased, diarrhea, dry mouth, onycholysis, alanine aminotransferase increased, alkaline phosphatase increased, sodium decreased, decreased appetite, albumin decreased, dysgeusia, hemoglobin decreased, dry skin, aspartate aminotransferase increased, magnesium decreased, dry eye, alopecia, palmar-plantar erythrodysesthesia syndrome, constipation, phosphate decreased, abdominal pain, calcium increased, nausea, and musculoskeletal pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Erdafitinib is indicated for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma that has:
- susceptible FGFR3 or FGFR2 genetic alterations, and
- progressed during or following at least one line of prior platinum-containing chemotherapy, including within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy
- Select patients for therapy based on an FDA-approved companion diagnostic for erdafitinib.
- This indication is approved under accelerated approval based on tumor response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Dosage
- Recommended initial dosage: 8 mg orally once daily with a dose increase to 9 mg daily if criteria are met.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding erdafitinib Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding erdafitinib Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness of erdafitinib in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding erdafitinib Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding erdafitinib Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
Ocular Disorders
- Erdafitinib can cause ocular disorders, including central serous retinopathy/retinal pigment epithelial detachment (CSR/RPED) resulting in visual field defect.
- CSR/RPED was reported in 25% of patients treated with erdafitinib, with a median time to first onset of 50 days. Grade 3 CSR/RPED, involving central field of vision, was reported in 3% of patients. CSR/RPED resolved in 13% of patients and was ongoing in 13% of patients at the study cutoff. CSR/RPED led to dose interruptions and reductions in 9% and 14% of patients, respectively and 3% of patients discontinued erdafitinib.
- Dry eye symptoms occurred in 28% of patients during treatment with erdafitinib and were Grade 3 in 6% of patients. All patients should receive dry eye prophylaxis with ocular demulcents as needed.
- Perform monthly ophthalmological examinations during the first 4 months of treatment and every 3 months afterwards, and urgently at any time for visual symptoms. Ophthalmological examination should include assessment of visual acuity, slit lamp examination, fundoscopy, and optical coherence tomography.
- Withhold erdafitinib when CSR occurs and permanently discontinue if it does not resolve within 4 weeks or if Grade 4 in severity. For ocular adverse reactions, follow the dose modification guidelines.
Hyperphosphatemia
- Increases in phosphate levels are a pharmacodynamic effect of erdafitinib. Hyperphosphatemia was reported as adverse reaction in 76% of patients treated with erdafitinib. The median onset time for any grade event of hyperphosphatemia was 20 days (range: 8 –116) after initiating erdafitinib. Thirty-two percent of patients received phosphate binders during treatment with erdafitinib.
- Monitor for hyperphosphatemia and follow the dose modification guidelines when required.
Embryo-Fetal Toxicity
- Based on the mechanism of action and findings in animal reproduction studies, erdafitinib can cause fetal harm when administered to a pregnant woman. In an embryo-fetal toxicity study, oral administration of erdafitinib to pregnant rats during the period of organogenesis caused malformations and embryo-fetal death at maternal exposures that were less than the human exposures at the maximum human recommended dose based on area under the curve (AUC). Advise pregnant women of the potential risk to the fetus. Advise female patients of reproductive potential to use effective contraception during treatment with erdafitinib and for one month after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with erdafitinib and for one month after the last dose.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of erdafitinib was evaluated in the BLC2001 study that included 87 patients with locally advanced or metastatic urothelial carcinoma which had susceptible FGFR3 or FGFR2 genetic alterations, and which progressed during or following at least one line of prior chemotherapy including within 12 months of neoadjuvant or adjuvant chemotherapy. Patients were treated with erdafitinib at 8 mg orally once daily; with a dose increase to 9 mg in patients with phosphate levels <5.5 mg/dL on Day 14 of Cycle 1. Median duration of treatment was 5.3 months (range: 0 to 17 months).
- The most common adverse reactions (ARs) including laboratory abnormalities (≥20%) were phosphate increased, stomatitis, fatigue, creatinine increased, diarrhea, dry mouth, onycholysis, alanine aminotransferase increased, alkaline phosphatase increased, sodium decreased, decreased appetite, albumin decreased, dysgeusia, hemoglobin decreased, dry skin, aspartate aminotransferase increased, magnesium decreased, dry eye, alopecia, palmar-plantar erythrodysesthesia syndrome, constipation, phosphate decreased, abdominal pain, calcium increased, nausea, and musculoskeletal pain. The most common Grade 3 or greater ARs (>1%) were stomatitis, nail dystrophy, palmar-plantar erythrodysesthesia syndrome, paronychia, nail disorder, keratitis, onycholysis, and hyperphosphatemia.
- An adverse reaction with a fatal outcome in 1% of patients was acute myocardial infarction.
- Serious adverse reactions occurred in 41% of patients including eye disorders (10%).
- Permanent discontinuation due to an adverse reaction occurred in 13% of patients. The most frequent reasons for permanent discontinuation included eye disorders (6%).
- Dosage interruptions occurred in 68% of patients. The most frequent adverse reactions requiring dosage interruption included hyperphosphatemia (24%), stomatitis (17%), eye disorders (17%), and palmar-plantar erythro-dysaesthesia syndrome (8%).
- Dose reductions occurred in 53% of patients. The most frequent adverse reactions for dose reductions included eye disorders (23%), stomatitis (15%), hyperphosphatemia (7%), palmar-plantar erythro-dysaesthesia syndrome (7%), paronychia (7%), and nail dystrophy (6%).
- Table 3 presents ARs reported in ≥10% of patients treated with erdafitinib at 8 mg once daily.


Postmarketing Experience
There is limited information regarding Erdafitinib Postmarketing Experience in the drug label.
Drug Interactions
Effect of Other Drugs on erdafitinib
- Table 5 summarizes drug interactions that affect the exposure of erdafitinib or serum phosphate level and their clinical management.

Effect of erdafitinib on Other Drugs
- Table 6 summarizes the effect of erdafitinib on other drugs and their clinical management.

Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- Based on the mechanism of action and findings in animal reproduction studies, erdafitinib can cause fetal harm when administered to a pregnant woman. There are no available data on erdafitinib use in pregnant women to inform a drug-associated risk. Oral administration of erdafitinib to pregnant rats during organogenesis caused malformations and embryo-fetal death at maternal exposures that were less than the human exposures at the maximum recommended human dose based on AUC. Advise pregnant women and females of reproductive potential of the potential risk to the fetus.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Animal Data
- In an embryo-fetal toxicity study, erdafitinib was orally administered to pregnant rats during the period of organogenesis. Doses ≥4mg/kg/day (at total maternal exposures <0.1% of total human exposures at the maximum recommended human dose based on AUC) produced embryo-fetal death, major blood vessel malformations and other vascular anomalies, limb malformations (ectrodactyly, absent or misshapen long bones), an increased incidence of skeletal anomalies in multiple bones (vertebrae, sternebrae, ribs), and decreased fetal weight.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Erdafitinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Erdafitinib during labor and delivery.
Nursing Mothers
- There are no data on the presence of erdafitinib in human milk, or the effects of erdafitinib on the breastfed child, or on milk production. Because of the potential for serious adverse reactions from erdafitinib in a breastfed child, advise lactating women not to breastfeed during treatment with erdafitinib and for one month following the last dose.
Pediatric Use
- Safety and effectiveness of erdafitinib in pediatric patients have not been established.
- In 4 and 13-week repeat-dose toxicology studies in rats and dogs, toxicities in bone and teeth were observed at an exposure less than the human exposure (AUC) at the maximum recommended human dose. Chondroid dysplasia/metaplasia were reported in multiple bones in both species, and tooth abnormalities included abnormal/irregular denting in rats and dogs and discoloration and degeneration of odontoblasts in rats.
Geriatic Use
- Of the 416 patients treated with erdafitinib in clinical studies, 45% were 65 years of age or older, and 12% were 75 years of age or older. No overall differences in safety or effectiveness were observed between these patients and younger patients
Gender
There is no FDA guidance on the use of Erdafitinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Erdafitinib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Erdafitinib in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Erdafitinib in patients with hepatic impairment.
Females of Reproductive Potential and Males
Pregnancy Testing
- Pregnancy testing is recommended for females of reproductive potential prior to initiating treatment with erdafitinib.
Contraception (Females)
- Erdafitinib can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with erdafitinib and for one month after the last dose.
Contraception (Males)
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment with erdafitinib and for one month after the last dose.
Infertility
- Based on findings from animal studies, erdafitinib may impair fertility in females of reproductive potential.
Immunocompromised Patients
There is no FDA guidance one the use of Erdafitinib in patients who are immunocompromised.
CYP2C9 Poor Metabolizers
- CYP2C9*3/*3 Genotype: Erdafitinib plasma concentrations were predicted to be higher in patients with the CYP2C9*3/*3 genotype. Monitor for increased adverse reactions in patients who are known or suspected to have CYP2C9*3/*3 genotype.
Administration and Monitoring
Administration
Patient Selection
- Select patients for the treatment of locally advanced or metastatic urothelial carcinoma with erdafitinib based on the presence of susceptible FGFR genetic alterations in tumor specimens as detected by an FDA-approved companion diagnostic.
- Information on FDA-approved tests for the detection of FGFR genetic alterations in urothelial cancer is available at: http://www.fda.gov/CompanionDiagnostics.
Recommended Dosage and Schedule
- The recommended starting dose of erdafitinib is 8 mg (two 4 mg tablets) orally once daily, with a dose increase to 9 mg (three 3 mg tablets) once daily based on serum phosphate (PO4) levels and tolerability at 14 to 21 days.
- Swallow tablets whole with or without food. If vomiting occurs any time after taking erdafitinib, the next dose should be taken the next day. Treatment should continue until disease progression or unacceptable toxicity occurs.
- If a dose of erdafitinib is missed, it can be taken as soon as possible on the same day. Resume the regular daily dose schedule for erdafitinib the next day. Extra tablets should not be taken to make up for the missed dose.
Dose Increase based on Serum Phosphate Levels
- Assess serum phosphate levels 14 to 21 days after initiating treatment. Increase the dose of erdafitinib to 9 mg once daily if serum phosphate level is < 5.5 mg/dL and there are no ocular disorders or Grade 2 or greater adverse reactions. Monitor phosphate levels monthly for hyperphosphatemia.
Dose Modifications for Adverse Reactions
- The recommended dose modifications for adverse reactions are listed in Table 1.

- Table 2 summarizes recommendations for dose interruption, reduction, or discontinuation of erdafitinib in the management of specific adverse reactions.

Monitoring
There is limited information regarding Erdafitinib Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Erdafitinib and IV administrations.
Overdosage
There is limited information regarding Erdafitinib overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
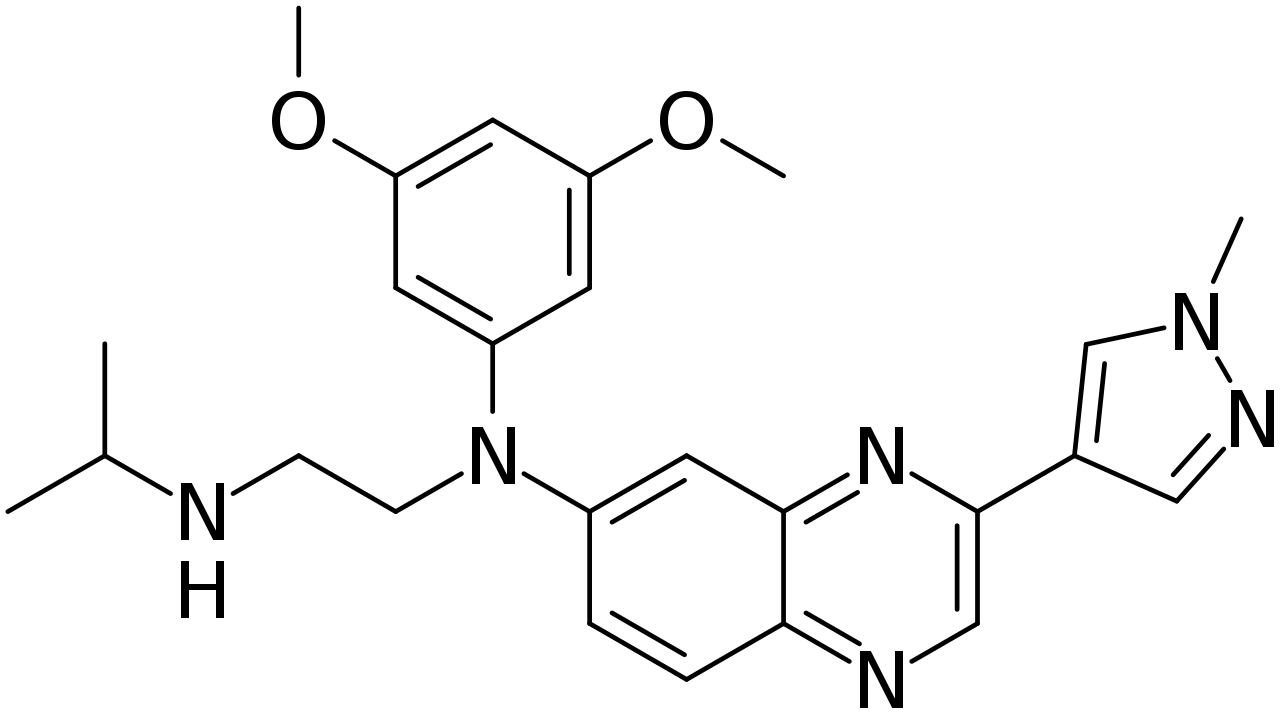
Erdafitinib
| |
| Systematic (IUPAC) name | |
| N′-(3,5-Dimethoxyphenyl)-N′-[3-(1-methylpyrazol-4-yl)quinoxalin-6-yl]-N-propan-2-ylethane-1,2-diamine | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| SMILES | & |
| Synonyms | JNJ-42756493 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Erdafitinib is a kinase inhibitor that binds to and inhibits enzymatic activity of FGFR1, FGFR2, FGFR3 and FGFR4 based on in vitro data. Erdafitinib also binds to RET, CSF1R, PDGFRA, PDGFRB, FLT4, KIT, and VEGFR2. Erdafitinib inhibited FGFR phosphorylation and signaling and decreased cell viability in cell lines expressing FGFR genetic alterations, including point mutations, amplifications, and fusions. Erdafitinib demonstrated antitumor activity in FGFR-expressing cell lines and xenograft models derived from tumor types, including bladder cancer.
Structure

Pharmacodynamics
Cardiac Electrophysiology
- Based on evaluation of QTc interval in an open-label, dose escalation and dose expansion study in 187 patients with cancer, erdafitinib had no large effect (i.e., > 20 ms) on the QTc interval.
Serum Phosphate
- Erdafitinib increased serum phosphate level as a consequence of FGFR inhibition. Erdafitinib should be increased to the maximum recommended dose to achieve target serum phosphate levels of 5.5–7.0 mg/dL in early cycles with continuous daily dosing.
- In erdafitinib clinical trials, the use of drugs which can increase serum phosphate levels, such as potassium phosphate supplements, vitamin D supplements, antacids, phosphate-containing enemas or laxatives, and medications known to have phosphate as an excipient were prohibited unless no alternatives exist. To manage phosphate elevation, phosphate binders were permitted. Avoid concomitant use with agents that can alter serum phosphate levels before the initial dose increase period based on serum phosphate levels.
Pharmacokinetics
- Following administration of 8 mg once daily, the mean (coefficient of variation [CV%]) erdafitinib steady-state maximum observed plasma concentration (Cmax), area under the curve (AUCtau), and minimum observed plasma concentration (Cmin) were 1,399 ng/mL (51%), 29,268 ng∙h/mL (60%), and 936 ng/mL (65%), respectively.
- Following single and repeat once daily dosing, erdafitinib exposure (maximum observed plasma concentration [Cmax] and area under the plasma concentration time curve [AUC]) increased proportionally across the dose range of 0.5 to 12 mg (0.06 to 1.3 times the maximum approved recommended dose). Steady state was achieved after 2 weeks with once daily dosing and the mean accumulation ratio was 4-fold.
Absorption
- Median time to achieve peak plasma concentration (tmax) was 2.5 hours (range: 2 to 6 hours).
Effect of Food
- No clinically meaningful differences with erdafitinib pharmacokinetics were observed following administration of a high-fat and high-calorie meal (800 calories to 1,000 calories with approximately 50% of total caloric content of the meal from fat) in healthy subjects.
Distribution
- The mean apparent volume of distribution of erdafitinib was 29 L in patients.
- Erdafitinib protein binding was 99.8% in patients, primarily to alpha-1-acid glycoprotein.
Elimination
- The mean total apparent clearance (CL/F) of erdafitinib was 0.362 L/h in patients.
- The mean effective half-life of erdafitinib was 59 hours in patients.
Metabolism
- Erdafitinib is primarily metabolized by CYP2C9 and CYP3A4. The contribution of CYP2C9 and CYP3A4 in the total clearance of erdafitinib is estimated to be 39% and 20% respectively. Unchanged erdafitinib was the major drug-related moiety in plasma, there were no circulating metabolites.
Excretion
- Following a single oral dose of radiolabeled erdafitinib, approximately 69% of the dose was recovered in feces (19% as unchanged) and 19% in urine (13% as unchanged).
Specific Populations
- No clinically meaningful trends in the pharmacokinetics of erdafitinib were observed based on age (21–88 years), sex, race, body weight (36–132 kg), mild (eGFR [estimated glomerular filtration rate, using modification of diet in renal disease equation] 60 to 89 mL/min/1.73 m2) or moderate (eGFR 30–59 mL/min/1.73 m2) renal impairment or mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN, or total bilirubin > 1.0–1.5 × ULN and any AST).
- The pharmacokinetics of erdafitinib in patients with severe renal impairment, renal impairment requiring dialysis, moderate or severe hepatic impairment is unknown.
Drug Interaction Studies
- Clinical Studies and Model-Based Approaches Strong CYP2C9 Inhibitors:
- Erdafitinib mean ratios (90% CI) for Cmax and AUCinf were 121% (99.9, 147) and 148% (120, 182), respectively, when co-administered with fluconazole, a strong CYP2C9 inhibitor and moderate CYP3A4 inhibitor, relative to erdafitinib alone.
- Strong CYP3A4 Inhibitors:
- Erdafitinib mean ratios (90% CI) for Cmax and AUCinf were 105% (86.7, 127) and 134% (109, 164), respectively, when co-administered with itraconazole (a strong CYP3A4 inhibitor and P-gp inhibitor) relative to erdafitinib alone.
- Strong CYP3A4/2C9 Inducers:
- Simulations suggested that rifampicin (a strong CYP3A4/2C9 inducer) may significantly decrease erdafitinib Cmax and AUC.
In Vitro Studies
- CYP Substrates:
- Erdafitinib is a time dependent inhibitor and inducer of CYP3A4. The effect of erdafitinib on a sensitive CYP3A4 substrate is unknown. Erdafitinib is not an inhibitor of other major CYP isozymes at clinically relevant concentrations.
- Transporters:
- Erdafitinib is a substrate and inhibitor of P-gp. P-gp inhibitors are not expected to affect erdafitinib exposure to a clinically relevant extent. Erdafitinib is an inhibitor of OCT2.
- Erdafitinib does not inhibit BCRP, OATP1B, OATP1B3, OAT1, OAT3, OCT1, MATE-1, or MATE-2K at clinically relevant concentrations.
- Acid-Lowering Agents:
- Erdafitinib has adequate solubility across the pH range of 1 to 7.4. Acid-lowering agents (e.g., antacids, H2-antagonists, proton pump inhibitors) are not expected to affect the bioavailability of erdafitinib.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility
- Carcinogenicity studies have not been conducted with erdafitinib.
- Erdafitinib was not mutagenic in a bacterial reverse mutation (Ames) assay and was not clastogenic in an in vitro micronucleus or an in vivo rat bone marrow micronucleus assay.
- Fertility studies in animals have not been conducted with erdafitinib. In the 3-month repeat-dose toxicity study, erdafitinib showed effects on female reproductive organs (necrosis of the ovarian corpora lutea) in rats at an exposure less than the human exposure (AUC) at maximum recommended human dose.
Clinical Studies
Urothelial Carcinoma with Susceptible FGFR Genetic Alterations
- Study BLC2001 (NCT02365597) was a multicenter, open-label, single-arm study to evaluate the efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma(mUC). Fibroblast growth factor receptor (FGFR) mutation status for screening and enrollment of patients was determined by a clinical trial assay (CTA). The efficacy population consists of a cohort of eighty-seven patients who were enrolled in this study with disease that had progressed on or after at least one prior chemotherapy and that had at least 1 of the following genetic alterations: FGFR3 gene mutations (R248C, S249C, G370C, Y373C) or FGFR gene fusions (FGFR3-TACC3, FGFR3-BAIAP2L1, FGFR2-BICC1, FGFR2-CASP7), as determined by the CTA performed at a central laboratory. Tumor samples from 69 patients were tested retrospectively by the QIAGEN therascreen® FGFR RGQ RT-PCR Kit, which is the FDA-approved test for selection of patients with mUC for erdafitinib.
- Patients received a starting dose of erdafitinib at 8 mg once daily with a dose increase to 9 mg once daily in patients whose serum phosphate levels were below the target of 5.5 mg/dL between days 14 and 17; a dose increase occurred in 41% of patients. Erdafitinib was administered until disease progression or unacceptable toxicity. The major efficacy outcome measures were objective response rate (ORR) and duration of response (DoR), as determined by blinded independent review committee (BIRC) according to RECIST v1.1.
- The median age was 67 years (range: 36 to 87 years), 79% were male, and 74% were Caucasian. Most patients (92%) had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Sixty-six percent of patients had visceral metastases. Eighty-four (97%) patients received at least one of cisplatin or carboplatin previously. Fifty-six percent of patients only received prior cisplatin-based regimens, 29% received only prior carboplatin-based regimens, and 10% received both cisplatin and carboplatin-based regimens. Three (3%) patients had disease progression following prior platinum-containing neoadjuvant or adjuvant therapy only. Twenty-four percent of patients had been treated with prior anti PD-L1/PD-1 therapy.
- Efficacy results are summarized in Table 7 and Table 8. Overall response rate was 32.2%. Responders included patients who had previously not responded to anti PD-L1/PD-1 therapy.


How Supplied
- Erdafitinib tablets are available in the strengths and packages listed below:
- 3 mg tablets: Yellow, round biconvex, film-coated, debossed with "3" on one side and "EF" on the other side.
- Bottle of 56-tablets with child resistant closure
- Bottle of 84-tablets with child resistant closure
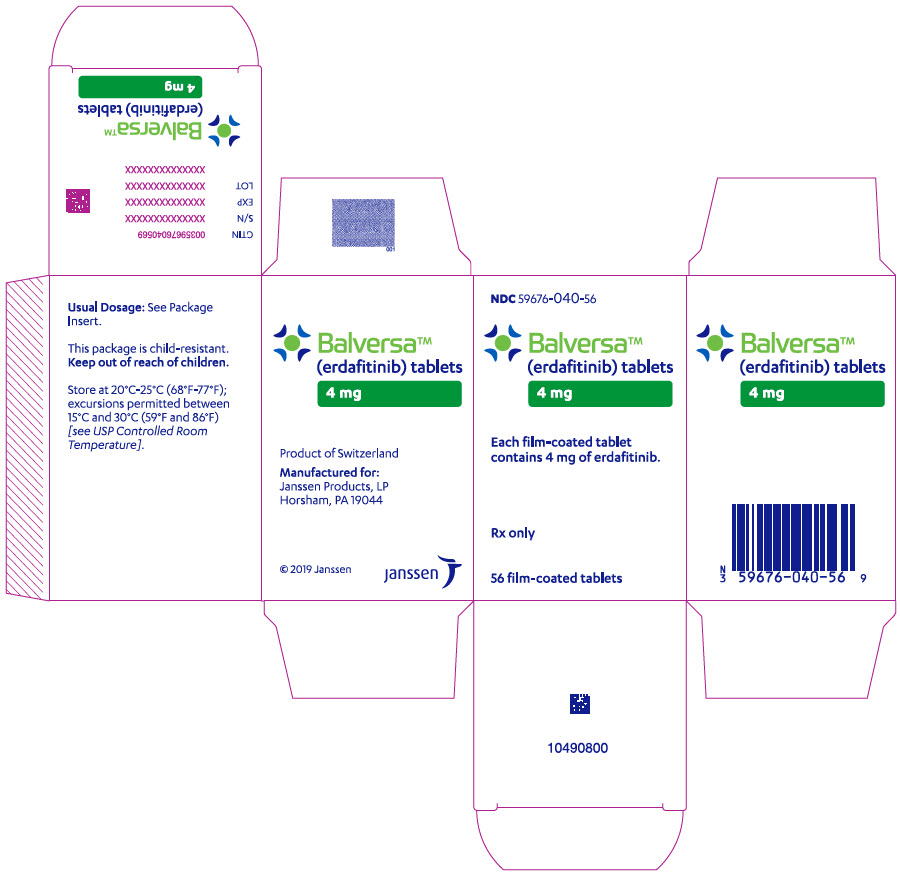
- 4 mg tablets: Orange, round biconvex, film-coated, debossed with "4" on one side and "EF" on the other side.
- Bottle of 28-tablets with child resistant closure
- Bottle of 56-tablets with child resistant closure
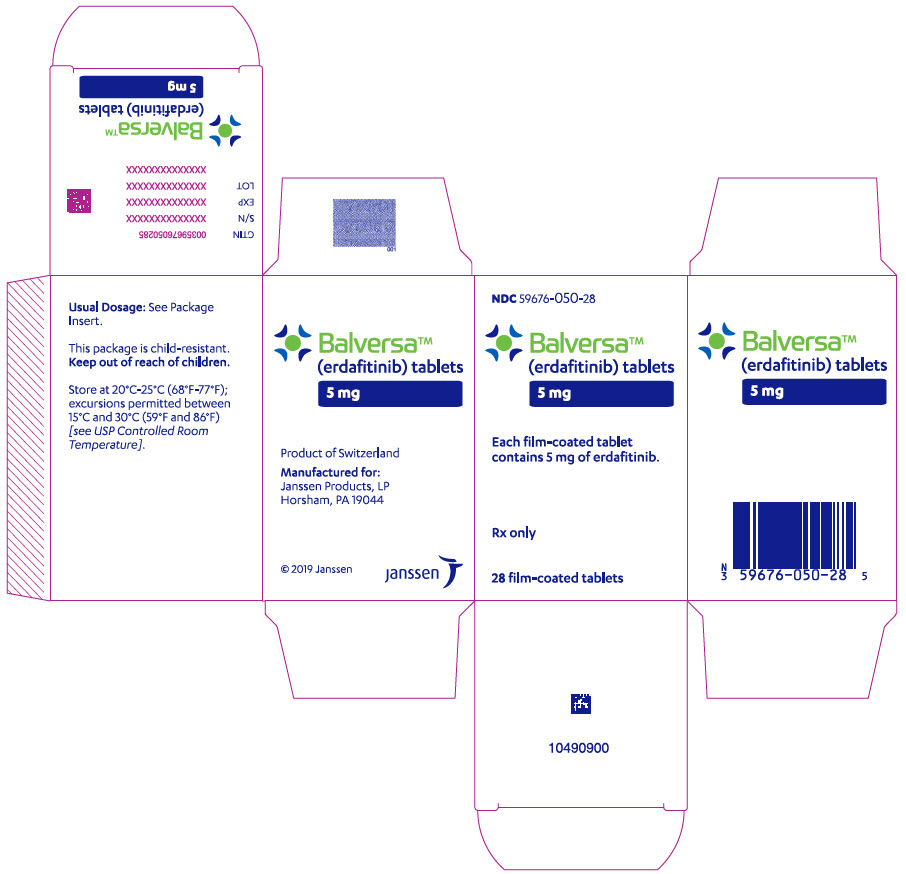
- 5 mg tablets: Brown, round biconvex, film-coated, debossed with "5" on one side and "EF" on the other side.
- Bottle of 28-tablets with child resistant closure
- 3 mg tablets: Yellow, round biconvex, film-coated, debossed with "3" on one side and "EF" on the other side.
Storage
- Store at 20°C–25°C (68°F–77°F); excursions permitted between 15°C and 30°C (59°F and 86°F).
Images
Drug Images
{{#ask: Page Name::Erdafitinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Erdafitinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information).
- FGFR genetic alterations: Advise patients that evidence of a susceptible FGFR3 or FGFR2 mutation or gene fusion within the tumor specimen is necessary to identify patients for whom treatment is indicated
- Ocular disorders: Advise patients to contact their healthcare provider if they experience any visual changes. In order to prevent or treat dry eyes, advise patients to use artificial tear substitutes, hydrating or lubricating eye gels or ointments frequently, at least every 2 hours during waking hours.
- Skin, mucous or nail disorders: Advise patients to contact their healthcare provider if they experience progressive or intolerable skin, mucous or nail disorders.
- Hyperphosphatemia: Advise patients that their healthcare provider will assess their serum phosphate level between 14 and 21 days of initiating treatment and will adjust the dose if needed. During this initial phosphate-assessment period, advise patients to avoid concomitant use with agents that can alter serum phosphate levels. Advise patients that, after the initial phosphate assessment period, monthly phosphate level monitoring for hyperphosphatemia should be performed during treatment with erdafitinib.
- Drug Interactions: Advise patients to inform their healthcare providers of all concomitant medications, including prescription medicines, over-the-counter drugs, and herbal products.
- Dosing Instructions: Instruct patients to swallow the tablets whole once daily with or without food. If vomiting occurs any time after taking erdafitinib, advise patients to take the next dose the next day.
- Missed dose: If a dose is missed, advise patients to take the missed as soon as possible. Resume the regular daily dose schedule for erdafitinib the next day. Extra tablets should not be taken to make up for the missed dose.
- Embryo-Fetal Toxicity: Advise pregnant women and females of reproductive potential of the potential risk to the fetus. Advise females to inform their healthcare providers of a known or suspected pregnancy. Advise female patients of reproductive potential to use effective contraception during treatment and for one month after the last dose of erdafitinib. Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for one month after the last dose of erdafitinib.
- Lactation: Advise females not to breastfeed during treatment with erdafitinib and for one month after the last dose.
Medication Guide

Precautions with Alcohol
Alcohol-Erdafitinib interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Erdafitinib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.