22q11.2 deletion syndrome pathophysiology
| https://https://www.youtube.com/watch?v=YdDs92gaWl8%7C350}} |
|
22q11.2 deletion syndrome Microchapters |
|
Differentiating 22q11.2 deletion syndrome from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
22q11.2 deletion syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of 22q11.2 deletion syndrome pathophysiology |
|
Risk calculators and risk factors for 22q11.2 deletion syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2] Ayushi Jain, M.B.B.S[3]
Overview
The main factor leading to DGS is the deletion of 22q11.2, which encodes over 90 genes. Researchers have not yet identified all of the genes that contribute to the features of 22q11.2 deletion syndrome. They have determined that the loss of one particular gene on chromosome 22, TBX1, is probably responsible for many of the syndrome's characteristic signs (such as heart defects, a cleft palate, distinctive facial features, and low calcium levels). A loss of this gene does not appear to cause learning disabilities, however. Additional genes in the deleted region are likely to contribute to the signs and symptoms of 22q11.2 deletion syndrome.
Pathophysiology
The main factor leading to DGS is the deletion of 22q11.2, which encodes over 90 genes. Because the signs and symptoms of 22q11.2 deletion syndrome are so varied, different groupings of features were once described as separate conditions. These included the velo-cardio-facial syndrome (also called Shprintzen's syndrome), DiGeorge syndrome, hearing loss with craniofacial syndromes and conotruncal anomaly face syndrome, thymic hypoplasia, cleft palate, psychiatric disorders, and hypocalcaemia. The acronym CATCH-22 (C = cardiac defects, A = abnormal facies, T = thymic hypoplasia, C = cleft palate, H = hypocalcemia from parathyroid aplasia, 22 = microdeletions in chromosome 22) is sometimes used, although it is widely rejected because of the negative connotations with Catch-22 meaning a 'no-win' situation.
In addition, some children with the 22q11.2 deletion were diagnosed with Opitz G/BBB syndrome and Cayler cardiofacial syndrome. Once the genetic basis for these disorders was identified, doctors determined that they were all part of a single syndrome with many possible signs and symptoms. To avoid confusion, this condition is usually called 22q11.2 deletion syndrome, a description based on its underlying genetic cause.
Genetics
Most people with 22q11.2 deletion syndrome are missing about 3 million base pairs (the building blocks of DNA) on one copy of chromosome 22 in each cell. This region contains about 30 genes, but many of these genes have not been well characterized. A small percentage of affected individuals have shorter deletions in the same region. This condition is often described as a contiguous gene deletion syndrome because a deletion in chromosome 22 leads to the loss of many genes.
CAUSE OF DELETION
The deletion is bracketed by low copy number repeats (LCRs).Four discrete blocks of LCRs are found in this region and each block is comprised of multiple repeats. These blocks are named LCR A-D, with A being the most proximal (Figure 1). The deletion typically arises via unequal meiotic exchange, facilitated by asynchronous replication at the site of the deletion.The asynchronous replication can be associated with mispairing of the LCR and subsequent unequal crossing over. This mechanism predicts that duplications and deletions would be found in equal numbers. The characteristic deletion of chromosome 22q11.2 is at least 10 times more common than the next most frequent human deletion syndrome, suggesting that these repeat blocks are inherently unstable.The LCRs on chromosome 22q11.2 are larger and more complex and have higher homology than any of the other LCRs in the genome associated with human chromosomal deletion syndromes.[1]
The syndrome is caused by genetic deletions (loss of a small part of the genetic material) found on the long arm of the 22nd chromosome. Some patients with similar clinical features may have deletions on the short arm of chromosome 10.
DiGeorge syndrome causes migration defects of neural crest-derived tissues, particularly affecting development of the third and fourth Branchial pouches (pharyngeal pouches). Also affected is the thymus gland; a mediastinal organ largely responsible for differentiation and induction of tolerance in T-cells. Impaired immune function results principally from this etiology.
Researchers have not yet identified all of the genes that contribute to the features of 22q11.2 deletion syndrome. They have determined that the loss of one particular gene on chromosome 22, TBX1, is probably responsible for many of the syndrome's characteristic signs (such as heart defects, a cleft palate, distinctive facial features, and low calcium levels). A loss of this gene does not appear to cause learning disabilities, however. Additional genes in the deleted region are likely to contribute to the signs and symptoms of 22q11.2 deletion syndrome.
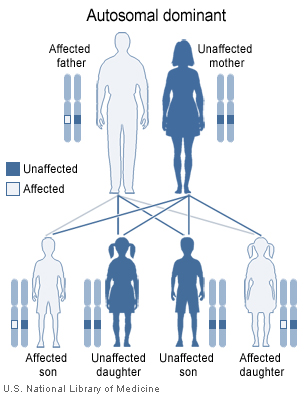
The 22q11.2 deletion syndrome can be inherited in an autosomal dominant manner. Almost all (about 93%) of cases have a de novo (new to the family) deletion of 22q11.2 but about 7% inherit the 22q11.2 deletion from a parent. Children of an individual with deletion 22q11.2 have a 50% chance of inheriting the 22q11.2 deletion. Prenatal testing, such as amniocentesis, is available for pregnancies determined to be at risk. Also pregnancies who have findings of congenital heart disease and/or cleft palate detected by ultrasound examination may be offered prenatal testing. Genetic counseling may be helpful for families who may have DiGeorge syndrome. Because most of the signs of this cluster of defects can also be inherited as autosomal recessive or x-linked traits, only genetic testing of both parents can determine with any certainty the likelihood these anomalies occurring in any subsequent children.
Associated Disorders
- Congenital heart disease (74% of individuals), particularly conotruncal malformations (tetralogy of Fallot, interrupted aortic arch, ventricular septal defect, and persistent truncus arteriosus)
- Immune deficiency
- Renal anomalies (37%)
- Autoimmune disorders
- Autism and Autism spectrum disorders
Thymus, parathyroid glands and heart derive from the same primitive embryonic structure and that is why these three organs are dysfunctioned together in this disease. Affected patients (usually children) are prone to yeast infections.
References
- ↑ McDonald-McGinn, Donna M. MS, CGC; Sullivan, Kathleen E. MD, PhD Chromosome 22q11.2 Deletion Syndrome (DiGeorge Syndrome/Velocardiofacial Syndrome), Medicine: January 2011 - Volume 90 - Issue 1 - p 1-18 doi: 10.1097/MD.0b013e3182060469
]