Wolff-Parkinson-White syndrome pathophysiology
|
Wolff-Parkinson-White syndrome Microchapters |
|
Differentiating Wolff-Parkinson-White syndrome from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Wolff-Parkinson-White syndrome pathophysiology On the Web |
|
Risk calculators and risk factors for Wolff-Parkinson-White syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
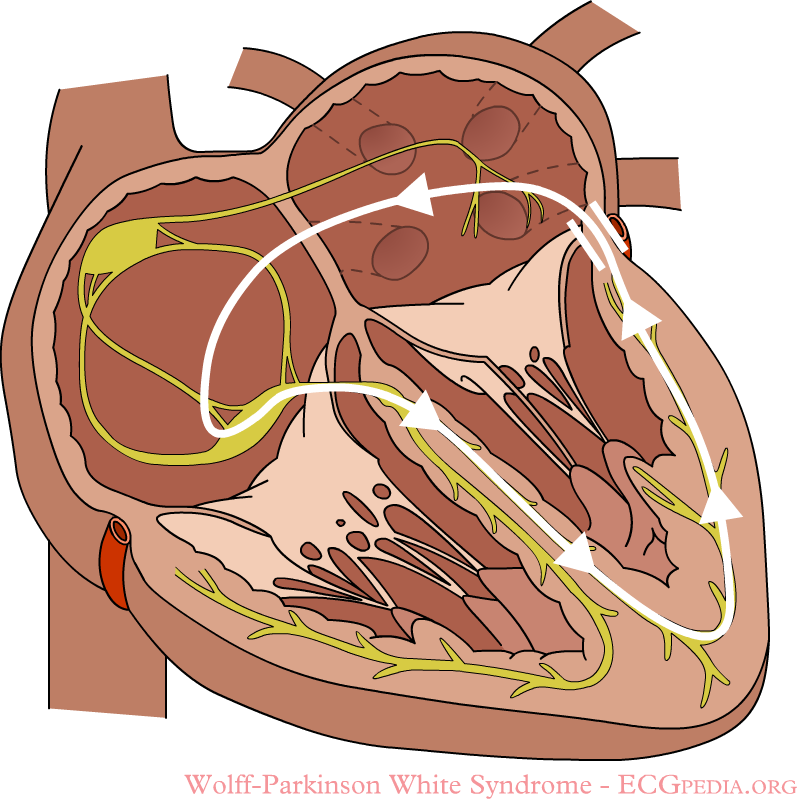
In normal individuals, electrical activity in the heart is initiated in the sinoatrial (SA) node (located in the right atrium), propagates to the atrioventricular (AV) node, and then through the bundle of His to the ventricles of the heart. Individuals with WPW have an accessory pathway, known as the bundle of Kent, that communicates between the atria and the ventricles. This accessory pathway does not share the rate-slowing properties of the AV node, and may conduct electrical activity at a significantly higher rate than the AV node. Extremely rapid heart rates may result in hemodynamic instability or cardiogenic shock, and in some cases, trigger ventricular fibrillation, a leading cause of sudden cardiac death.
Pathophysiology
- Electrical activity in the normal human heart is initiated when a cardiac action potential arises in the sinoatrial (SA) node, which is located in the right atrium. From there, the electrical stimulus is transmitted via internodal pathways to the atrioventricular (AV) node. After a brief delay at the AV node, the stimulus is conducted through the bundle of His to the left and right bundle branches and then to the Purkinje fibers and the endocardium at the apex of the heart, then finally to the ventricular myocardium.
- The AV node serves an important function as a "gatekeeper", limiting the electrical activity that reaches the ventricles. In situations where the atria generate excessively rapid electrical activity (such as atrial fibrillation or atrial flutter), the AV node limits the number of signals conducted to the ventricles. For example, if the atria are electrically activated at 300 beats per minute, half those electrical impulses may be blocked by the AV node, so that the ventricles are stimulated at only 150 beats per minute—resulting in a pulse of 150 beats per minute. Another important property of the AV node is that it slows down individual electrical impulses. This is manifested on the electrocardiogram as the PR interval (the time from electrical activation of the atria to electrical activation of the ventricles), which is usually shortened to less than 120 milliseconds in duration.
- Individuals with WPW have an accessory pathway that communicates between the atria and the ventricles, in addition to the AV node. This accessory pathway is known as the bundle of Kent. The bundle of Kent is an abnormal extra or accessory conduction pathway between the atria and ventricles that is present in a small percentage (between 0.1% and 0.3%) of the general population.[1][2] This pathway may communicate between the left atrium and the left ventricle, in which case it is termed a "type A pre-excitation", or between the right atrium and the right ventricle, in which case it is termed a "type B pre-excitation".[3] Problems arise when this pathway creates an electrical circuit that bypasses the AV node. The AV node is capable of slowing the rate of conduction of electrical impulses to the ventricles, whereas the bundle of Kent lacks this capability. When an aberrant electrical connection is made via the bundle of Kent, tachycardia may therefore result.
- This accessory pathway does not share the rate-slowing properties of the AV node, and may conduct electrical activity at a significantly higher rate than the AV node. For instance, in the example above, if an individual had an atrial rate of 300 beats per minute, the accessory bundle may conduct all the electrical impulses from the atria to the ventricles, causing the ventricles to contract at 300 beats per minute. Extremely rapid heart rates such as this may result in hemodynamic instability or cardiogenic shock. In some cases, the combination of an accessory pathway and cardiac arrhythmia can trigger ventricular fibrillation, a leading cause of sudden cardiac death.
Genetics
WPW syndrome has been identified in to have a genetic background as 0.55% of degree relatives of patients with WPW presents the disease. Missgene mutations (single nucelotide changes) in the gene PAKAG2 have been found in families with WPW. PAKAG2 encodes for the protein AMP-activated protein kinase (AMPK) gamma-2 subunit. AMPK, among other physiological functions, decreases glycogen synthesis, glycogen is abundant in the hearts conduction system, therefore an excesive acumulation of glycogen will prevent the accessory pathway to close. It has been reported that patients with familial WPW usualy have increased amounts of glycogen in the myocardial tissue. Patients with this disease, along with the preexcitations, they present myocardial hypertrophy and AV block. Nevertheless, WPW syndrome is usualy sporadic in origin and a small percentage from all csases have familial origin which presents as an autosomal dominant form.[4]
Associated Conditions
WPW syndrome is associated with the following disorders:
- Ebstein's anomaly[5][6][7]
- Mitral valve prolapse: This cardiac disorder, if present, is associated with left-sided accessory pathways.[8]
- Hypertrophic cardiomyopathy: This disorder is associated with familial/inherited form of WPW syndrome.[9]
References
- ↑ Sorbo MD, Buja GF, Miorelli M, Nistri S, Perrone C, Manca S, Grasso F, Giordano GM, Nava A (1995). "The prevalence of the Wolff–Parkinson–White syndrome in a population of 116,542 young males". Giornale Italiano di Cardiologia (in Italian). 25 (6): 681–7. PMID 7649416.
- ↑ Munger TM, Packer DL, Hammill SC, Feldman BJ, Bailey KR, Ballard DJ, Holmes DR Jr, Gersh BJ (1993). "A population study of the natural history of Wolff–Parkinson–White syndrome in Olmsted County, Minnesota, 1953–1989". Circulation. 87 (3): 866–73. doi:10.1161/01.CIR.87.3.866. PMID 8443907.
- ↑ americanheart.org Atrial and Ventricular Depolarization Changes Last updated 11/24/2008.
- ↑ Sidhu, J.; Roberts, R. (2003). "Genetic basis and pathogenesis of familial WPW syndrome". Indian Pacing Electrophysiol J. 3 (4): 197–201. PMID 16943919.
- ↑ Rao MP, Panduranga P, Al-Mukhaini M, Al-Jufaili M (2012). "Ebstein anomaly in an adult presenting with wide QRS tachycardia: diagnostic and therapeutic dilemmas". Am J Emerg Med. 30 (5): 834.e1–4. doi:10.1016/j.ajem.2011.03.001. PMID 21570234. Unknown parameter
|month=ignored (help) - ↑ Bayar N, Canbay A, Uçar O, Aydoğdu S, Diker E (2010). "[Association of Gerbode-type defect and Wolff-Parkinson-White syndrome with Ebstein's anomaly]". Anadolu Kardiyol Derg (in Turkish). 10 (1): 88–90. PMID 20150013. Unknown parameter
|month=ignored (help) - ↑ Legius B, Van De Bruaene A, Van Deyk K; et al. (2010). "Behavior of Ebstein's anomaly: single-center experience and midterm follow-up". Cardiology. 117 (2): 90–5. doi:10.1159/000318041. PMID 20924185.
- ↑ Savini E, Capone PL (1994). "[Wolff-Parkinson-White, a study on the prevalence of the site of accessory pathways: relations between stability of pre-excitation, symptoms, cardiac arrhythmias and association of mitral valve prolapse with localization of pre-excitation]". Minerva Cardioangiol (in Italian). 42 (7–8): 339–43. PMID 7970027.
- ↑ Kruchina TK, Vasichkina ES, Egorov DF, Tatarskiĭ BA (2012). "[Asymptomatic ventricular pre-excitation in children: a 17 year follow-up study]". Kardiologiia (in Russian). 52 (5): 30–6. PMID 22839583.