Wolff-Parkinson-White syndrome pathophysiology
|
Wolff-Parkinson-White syndrome Microchapters |
|
Differentiating Wolff-Parkinson-White syndrome from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Wolff-Parkinson-White syndrome pathophysiology On the Web |
|
Risk calculators and risk factors for Wolff-Parkinson-White syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
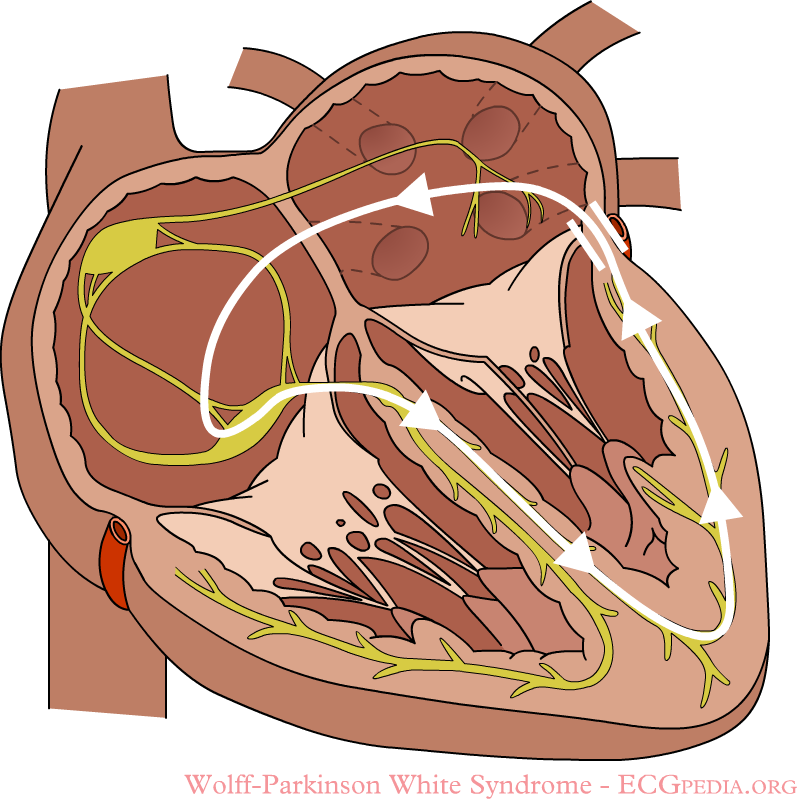
In normal individuals, electrical activity in the heart is initiated in the sinoatrial (SA) node (located in the right atrium), propagates to the atrioventricular (AV) node, and then through the bundle of His to the ventricles of the heart. (See electrical conduction system of the heart).
Pathophysiology
- The AV node slows the conduction of the impulses coming from the SA node, in that way the atrial contraction is completed before the ventricular contraction begins.
- In that way, if the impulses form the SA node increases (as in atrial fibrillation or atrial flutter) the AV node will slow the conduction to the ventricles. With this property of th AV node, if the atrium has a frequency of 300 beats per minute, only half of the impulses will reach the ventricles generating a heart rate of 150 beats per minute.
- The passage of the impulses through the AV node is manifested on the ECG as the PR interval; the period of time between the contraction of the atria and the contraction of the ventricles.
- In patients with WPW, an accessory pathway connects the atria and the ventricles in addition to the AV node, this accessory pathway is known as the bundle of Kent. This accessory pathway serves as a by-pass to the AV node as it does not have the capacity of of slowing the impulse that the AV node has, therefore it transmits impulses at higher rates.
- Using the example above, a patient with an atrial rate of 300 beats per minute with an accessory bundle may conduct all the electrical impulses with the ability of generating a ventricular rate of 300 beats per minute.
Genetics
WPW syndrome has been identified in to have a genetic backgournd as 0.55% of degree relatives of patients with WPW presents the disease. Missgene mutations (single nucelotide changes) in the gene PAKAG2 have been found in families with WPW. PAKAG2 encodes for the protein AMP-activated protein kinase (AMPK) gamma-2 subunit. AMPK, among other physiological functions, decreases glycogen synthesis, glycogen is abundant in the hearts conduction system, therefore an excesive acumulation of glycogen will prevent the accessory pathway to close. It has been reported that patients with familial WPW usualy have increased amounts of glycogen in the myocardial tissue. Patients with this disease, along with the preexcitations, they present myocardial hypertrophy and AV block. Nevertheless, WPW syndrome is usualy sporadic in origin and a small percentage from all csases have familial origin which presents as an autosomal dominant form.[1]
Associated Conditions
Associated disorders when present are most commonly associated with right-sided accessory pathway than left-sided pathways. WPW syndrome is associated with the following disorders:
- Ebstein's anomaly[2][3][4]
- Mitral valve prolapse: This cardiac disorder, if present, is associated with left-sided accessory pathways[5].
- Hypertrophic cardiomyopathy: This disorder is associated with familial/inherited form of WPW syndrome[6].
References
- ↑ Sidhu, J.; Roberts, R. (2003). "Genetic basis and pathogenesis of familial WPW syndrome". Indian Pacing Electrophysiol J. 3 (4): 197–201. PMID 16943919.
- ↑ Rao MP, Panduranga P, Al-Mukhaini M, Al-Jufaili M (2012). "Ebstein anomaly in an adult presenting with wide QRS tachycardia: diagnostic and therapeutic dilemmas". Am J Emerg Med. 30 (5): 834.e1–4. doi:10.1016/j.ajem.2011.03.001. PMID 21570234. Unknown parameter
|month=ignored (help) - ↑ Bayar N, Canbay A, Uçar O, Aydoğdu S, Diker E (2010). "[Association of Gerbode-type defect and Wolff-Parkinson-White syndrome with Ebstein's anomaly]". Anadolu Kardiyol Derg (in Turkish). 10 (1): 88–90. PMID 20150013. Unknown parameter
|month=ignored (help) - ↑ Legius B, Van De Bruaene A, Van Deyk K; et al. (2010). "Behavior of Ebstein's anomaly: single-center experience and midterm follow-up". Cardiology. 117 (2): 90–5. doi:10.1159/000318041. PMID 20924185.
- ↑ Savini E, Capone PL (1994). "[Wolff-Parkinson-White, a study on the prevalence of the site of accessory pathways: relations between stability of pre-excitation, symptoms, cardiac arrhythmias and association of mitral valve prolapse with localization of pre-excitation]". Minerva Cardioangiol (in Italian). 42 (7–8): 339–43. PMID 7970027.
- ↑ Kruchina TK, Vasichkina ES, Egorov DF, Tatarskiĭ BA (2012). "[Asymptomatic ventricular pre-excitation in children: a 17 year follow-up study]". Kardiologiia (in Russian). 52 (5): 30–6. PMID 22839583.