Wolff-Parkinson-White syndrome
| Wolff-Parkinson-White syndrome | ||
| ICD-10 | I45.6 | |
|---|---|---|
| ICD-9 | 426.7 | |
| OMIM | 194200 | |
| DiseasesDB | 14186 | |
| eMedicine | emerg/644 med/2417 | |
| MeSH | C14.280.067.780.977 | |
| Cardiology Network |
 Discuss Wolff-Parkinson-White syndrome further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Wolff-Parkinson-White syndrome (WPW) is a syndrome of pre-excitation of the ventricles of the heart due to an accessory pathway known as the Bundle of Kent. This accessory pathway is an abnormal electrical communication from the atria to the ventricles.
The incidence of WPW syndrome is between 0.1 and 3% of the general population.[1][2][3]
While the vast majority of individuals with WPW syndrome remain asymptomatic throughout their entire lives, there is a risk of sudden death associated with the syndrome. Sudden death due to WPW syndrome is rare (incidence of less than 0.6%[3][4]), and is due to the effect of the accessory pathway on tachyarrhythmias in these individuals.
History
Described more than 50 yrs ago and named for John Parkinson, Paul Dudley White (Paul Dudley White is a M.G.H. physician, evaluated a series of 11 healthy young patients who had attacks of paroxysmal tachycardias in the presence of an EKG which showed a bundle branch block pattern with a short PR interval), and Louis Wolff.[5][6]
Pathophysiology
In normal individuals, electrical activity in the heart is initiated in the sinoatrial (SA) node (located in the right atrium), propagates to the atrioventricular (AV) node, and then through the bundle of His to the ventricles of the heart. (See electrical conduction system of the heart).
The AV node acts as a gatekeeper, limiting the electrical activity that reaches the ventricles of the heart. This function of the AV node is important, because if the signals generated in the atria of the heart were to increase in rate (as they do during atrial fibrillation or atrial flutter), the AV node will limit the electrical activity that conducts to the ventricles. For instance, if the atria are electrically activated at 300 beats per minute, half those electrical impulses are blocked by the AV node, so that the ventricles are activated at 150 beats per minute (giving a pulse of 150 beats per minute). Another important property of the AV node is that it slows down individual electrical impulses. This is manifest on the ECG as the PR interval, the time from activation of the atria (manifest as the P wave) and activation of the ventricles (manifest as the QRS complex).
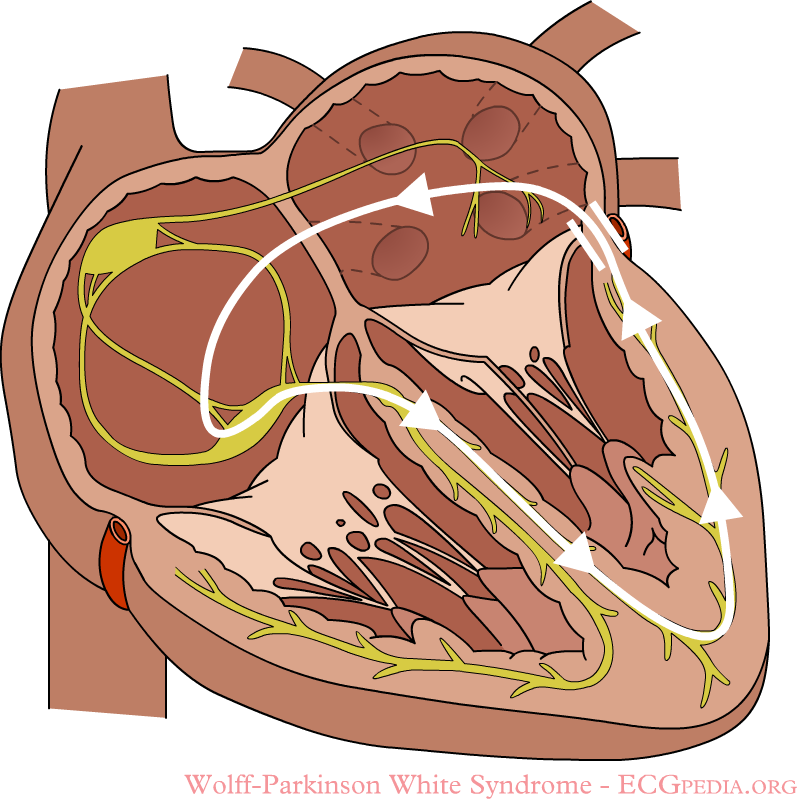
Individuals with WPW syndrome have an accessory pathway that connects the atria and the ventricles, in addition to the AV node. This accessory pathway is known as the bundle of Kent. This accessory pathway does not share the rate-slowing properties of the AV node, and may conduct electrical activity at a significantly higher rate than the AV node. For instance, in the example above, if an individual had an atrial rate of 300 beats per minute, the accessory bundle may conduct all the electrical impulses from the atria to the ventricles, causing the ventricles to activate at 300 beats per minute. Extremely fast heart rates are potentially dangerous, and can cause hemodynamic instability. In some cases, the combination of an accessory pathway and cardiac arrhythmias can trigger ventricular fibrillation, a leading cause of sudden cardiac death.
Diagnosis
WPW syndrome is commonly diagnosed on the basis of the surface ECG in an asymptomatic individual. In this case it is manifested as a delta wave, which is a slurred upstroke in the QRS complex that is associated with a short PR interval. The short PR interval and slurring of the QRS complex is actually the impulse making it through to the ventricles prematurely (across the accessory pathway) without the usual delay experienced in the AV node.
If the patient experiences episodes of atrial fibrillation, the ECG will show a rapid polymorphic wide-complex tachycardia (without turning of the points). This combination of atrial fibrillation and WPW is considered dangerous, and most antiarrhythmic drugs are contraindicated.
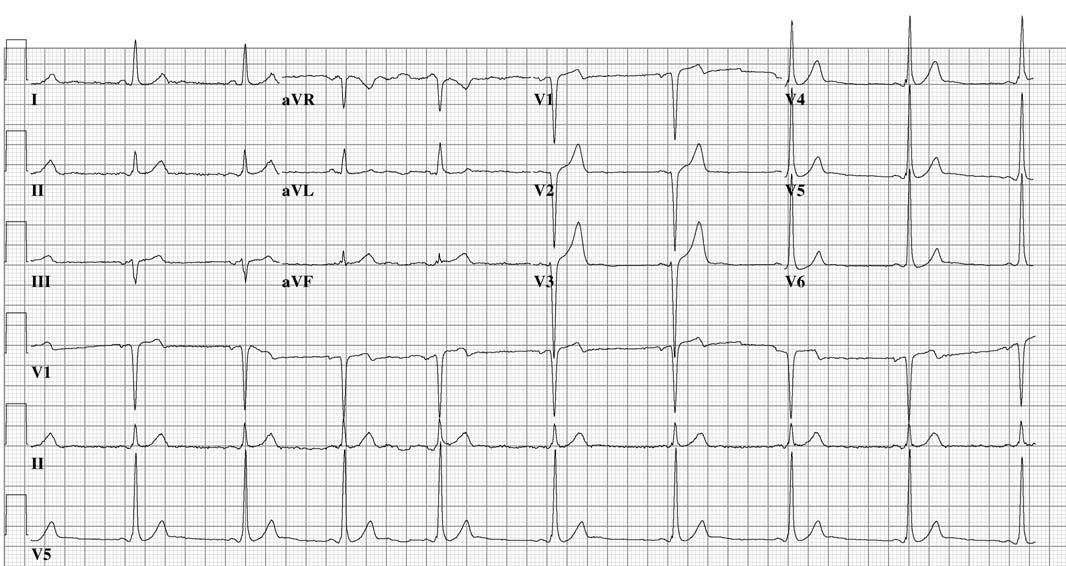
When an individual is in normal sinus rhythm, the ECG characteristics of WPW syndrome are a short PR interval, widened QRS complex (greater than 120 ms in length) with slurred upstroke of the QRS complex, and secondary repolarization changes reflected in ST segment-T wave changes.
In individuals with WPW syndrome, electrical activity that is initiated in the SA node travels through the accessory pathway as well as through the AV node to activate the ventricles via both pathways. Since the accessory pathway does not have the impulse slowing properties of the AV node, the electrical impulse first activates the ventricles via the accessory pathway, and immediately afterwards via the AV node. This gives the short PR interval and slurred upstroke to the QRS complex known as the delta wave.
Patients with WPW often exhibit more than one accessory pathway, and in some patients as many as eight additional abnormal pathways can be found. This has been seen in individuals with Ebstein's anomaly.
Wolff-Parkinson-White syndrome is sometimes associated with Leber's hereditary optic neuropathy (LHON), a form of mitochondrial disease.[7]
-
One beat from a rhythm strip in V2 demonstrating characteristic findings in WPW syndrome. Note the characteristic delta wave (subtler here than in some cases), the short PR interval of 0.08 seconds, and the long QRS complex at 0.12 seconds.
The EKG in WPW
- Two pathways between the atrium and the ventricle are present.
- There is a shortened PR interval
- PR less than 0.12 seconds
- in most cases it varies between 0.08 and 0.11 seconds
- A wide QRS with a delta wave.
- The delta wave occurs as the ventricle is activated first via the accessory pathway (AP) and then normal activation follows down the normal pathway.
- the duration of the delta wave is 0.03 to 0.06 seconds
- The pattern of ventricular activation is determined by several factors:
- the location of the accessory pathway: The closer the accessory pathway to the SA node, the quicker the impulse will reach the atrial insertion site of the AP. In contrast, in those patients in whom the AP is located in the far lateral region of the left ventricle, contribution to the AP during NSR may be minimal.
- the intra-atrial conduction time: Left atrial pathology will prolong the time necessary to reach the left sided AP, drugs can also prolong the time to reach a left-sided pathway.
- the conduction time over the accessory pathway: The conduction time over the AP depends on the length of the AP and velocity with which the impulse is conducted. Investigators have found that the accessory pathway may vary in length from 1 to 10 mm.
- the AV conduction time over the normal AV nodal-His-Purkinje pathway
- Secondary T wave changes:
- Concealed bypass tracts:
- If the accessory pathway's contribution to ventricular activation is minimal because of the coincidental arrival of the excitation wavefront over the normal pathway, then this should not be called a concealed accessory pathway.
- Concealed accessory pathways are those that conduct in a retrograde fashion (ventriculoatrial) only.
- Antegrade conduction in these patients is absent because the refractory period of the AP in the antegrade direction is longer than the sinus cycle length.
- when a recurrent tachycardia occurs in association with such concealed bypass, the conduction is called concealed WPW syndrome
- are usually located on the left side of the cardiac chambers
- consider this if during the tachycardia there is a negative P wave in lead V1, if there is a P wave after the QRS complex
- Findings are intermittent in 1/2 the cases
-
Wolf Parkinson White Left Posterior Pathway
-
Wolf Parkinson White Syndrome Posteroseptal Pathway
Incidence
- Somewhere between 0.1 and 3 per 1000 EKGs.
- May be underdiagnosed given the presence of left sided APs which may be silent during NSR.
- The incidence of tachyarrhythmias in patients with WPW has been estimated to be somewhere between 12% and 80%.
- WPW may be the most prevalent cause of paroxysmal regular supraventricular tachycardia One series found that 57% of patients c psvt were subsequently found to have WPW syndrome on the resting NSR EKG. If the attack occurs before the age of 21, then 73% of the patients had WPW. In patients > 21 years old, then WPW syndrome was found in 48% of patients with PSVT. (Wellens et al 1981).
EKG Classification
- Type A:
- Prominent R wave in lead V1 and V2.
- It has been found at EP studies that these patients have early activation of the left ventricle.
- Generally V1 shows either a notched R wave or RS or Rsr' deflection
- Mimics a posterior MI, RVH
- Type B:
- Prominent S wave deflection in the right precordial leads, and upright R waves in the lateral precordial leads.
- EP studies have showed that this form of WPW syndromes is due to early activation of the lateral aspect of the right ventricle
- This form is more common.
- May resemble an abnormal Q wave in the right precordial leads and be mistaken for an anterior MI
- In both type A and B there may be abnormal q waves in leads 2, 3 and aVF.
Determining the location of the accessory pathway
| Check lead V1 | |||||
| negative delta wave in V1 = right ventricle | positive delta wave om V1= left ventricle | ||||
| Negative delta wave and QRS in II, III, AVF | Left axis | Inferior axis | Negative delta wave and QRS in II, III, AVF | isoelectric or negative delta I, AVL, V5, V6 | |
| Posteroseptal | Right free wall | Anteroseptal | Posteroseptal | Lateral | |
Oversimplification
Histological studies have found that the AP fibers may insert in the septum and not the free wall as above. The location of AP may be impossible to determine in NSR, as it can be complicated by the existence of more than one AP in some patients, the coexistence of congenital lesions, the occasional superimposition of the P wave on the initial portion of the delta wave, and differences in the activation depending on whether the AP is epicardially or endocardially located.
Associated Cardiovascular Abnormalities
- Type B is found in 5% to 25% of the reported cases of Ebstein's dz. Suspect this if there is Type B WPW with RBBB.
- Also been found in patients with corrected transposition of the great arteries, tricuspid atresia, endocardial fibroelastosis, MVP, cardiomyopathies (hypertrophic obstructive and congestive).
Clinical Manifestations
- The most common form of paroxysmal tachycardia in these patients is a circus movement tachycardia (CMT) incorporating the AP.
- The CMT utilizes the following structures: the AV node, the His-Purkinje system, the ventricular myocardium (from the terminal portion of the His system to the ventricular end of the AP), the AP itself, and the atrial myocardium itself from the atrial insertion of the AP to the AV node.
- This circuit can conduct in both directions:
- Type I A CMT:
- This is the usual form of the CMT in patients with WPW.
- Is antegrade through the AV node, VA conduction through the AP.
- The QRS complex during the tachycardia shows either normal intraventricular conduction or typical bundle branch block configuration.
- Sx: Palpitations (97%), dyspnea (57%), anginal pain (56%), perspiration (55%), fatigue (41%), anxiety (30%), dizziness (30%),polyuria (26%).
- this is also called orthodromic reentrant tachycardia
- there is no delta wave
- the rate is 140 to 250 bpm
- it is faster than the rate of tachycardia due to reentry in the AV node
- often triggered by a PAC
- Type I B CMT:
- anterograde down the accessory pathway, retrograde down the AVN-His pathway.
- the QRS is widened
- this form is rarer
- Type II CMT (intra-AV nodal):
- anterograde pathway is an AV nodal slow pathway, the retrograde pathway is an AV nodal fast pathway.
- No evidence of ventricular pre-excitation during the tachycardia.
- Type III CMT (uses two accessory pathways):
- Conducts anterograde down one accessory pathway and retrograde up a second accessory pathway.
- These patients can also experience atrial tachycardias and ventricular tachycardias.
- Type I A CMT:
Atrial Fibrillation in WPW
Can cause life-threatening ventricular rates due to the exclusive AV conduction over the accessory pathway.
- reduces cardiac output.
- may degenerate into VF, particularly in those with multiple bypass tracts.
- The only marker identified for degeneration into VF in the literature was the occurrence of RR intervals equal to or less than 205 msec during the a fib
- Seen in 78 of 256 of Wellen's patients with WPW. Reported incidence is 20 to 35% in other studies.
- The degree of ventricular preexcitation observed in the EKG during NSR bears no relationship whatsoever to the risk of developing life-threatening ventricular rates during the a.fib.
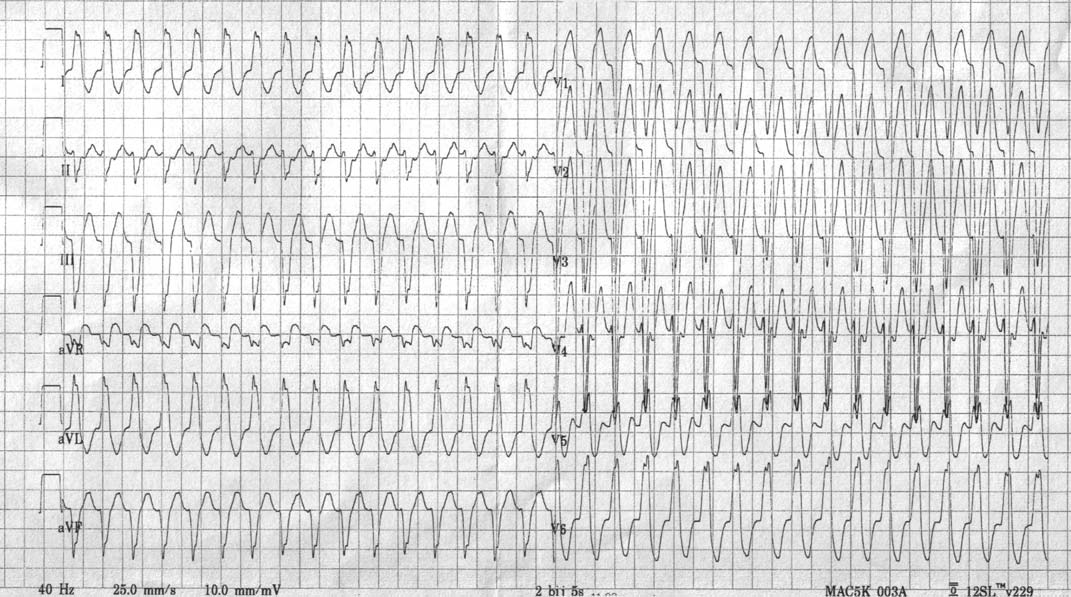
- The QRS complexes are wide and bizarre as a result of preexcitation.
- The ventricular rate is 220 to 360 beats per minute due to the short effective refractory period of the accessory pathway.
- It is often mistaken for VT.
- If the atrial rate in atrial fibrillation is greater than 200 BPM then suspect this. The rhythm will also be grossly irregular if it is due to atrial fibrillation. Such a rapid rate would be unusual if it were due to conduction by way of the normal AV conduction system.
-
Louis Wolff, Sir John Parkinson and Paul Dudley, who discovered the phenomenon that later would be called the WPW syndrome.
-
The upstroke of the QRS-complex is 'slurred', resulting in a delta-wave (arrow).
-
A atrioventricular tachycardia through the accessory bundle. The electrical signal travels from the ventricles via the accessory bundle to the atria and returns to the ventricles via the AV node.
-
Delta waves in a patient with Wolff-Parkinson-White Syndrome (WPW)
-
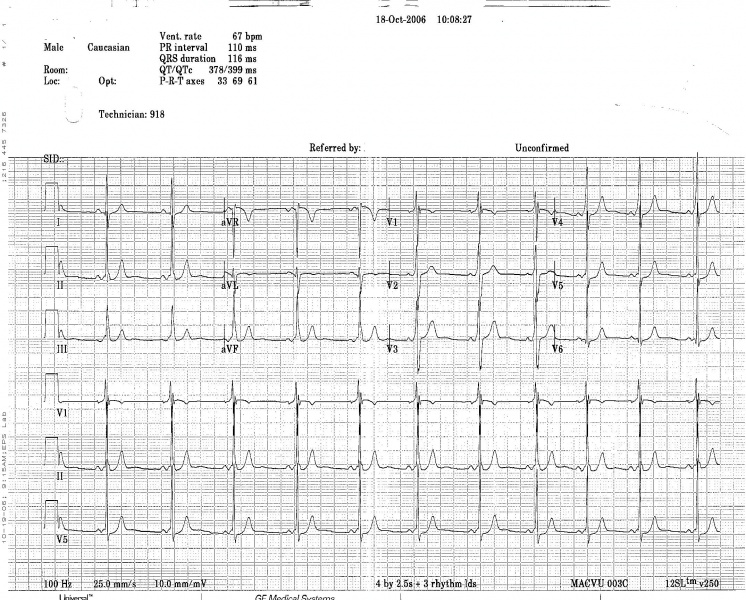
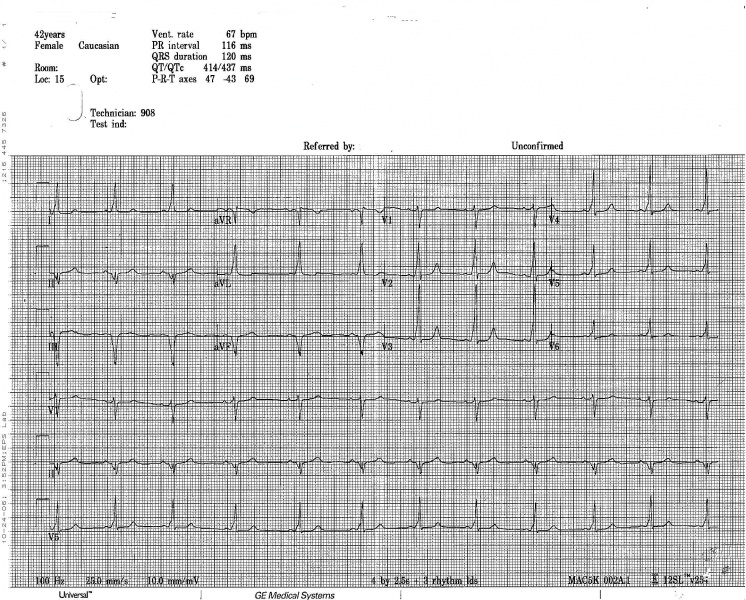
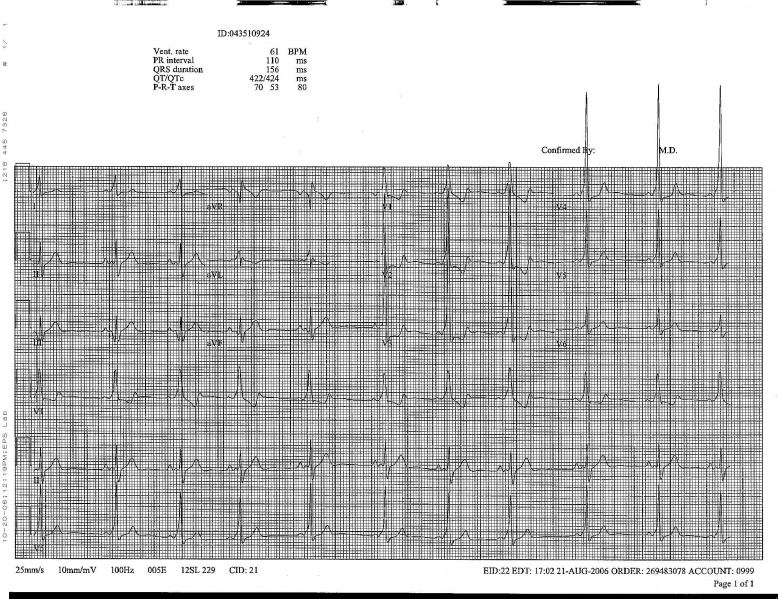
WPW on a 12 lead ECG.
-
Another example of WPW on a 12 lead ECG.
-
12 lead EKG: Wolff Parkinson White Syndrome
-
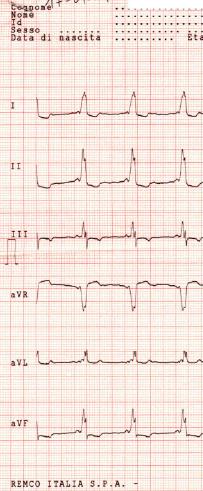
12 lead EKG: Wolff Parkinson White Syndrome Type I. Courtesy of Dr Jose Ganseman
-
12 lead EKG: Wolff Parkinson White Syndrome Type I. Courtesy of Dr Jose Ganseman
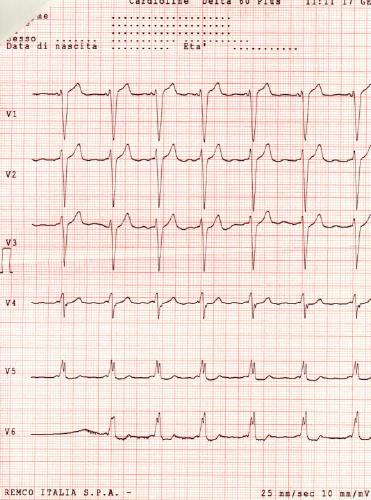
-
12 lead EKG: Wolff Parkinson White Syndrome Type I. Courtesy of Dr Jose Ganseman
-
12 lead EKG: Wolff Parkinson White Syndrome Type II. Courtesy of Dr Jose Ganseman
-
12 lead EKG: Wolff Parkinson White Syndrome Type II. Courtesy of Dr Jose Ganseman
-
12 lead EKG: Wolff Parkinson White Syndrome Type II. Courtesy of Dr Jose Ganseman
-
12 lead EKG: Wolff Parkinson White Syndrome Type II. Courtesy of Dr Jose Ganseman
-
12 lead EKG: Wolff Parkinson White Syndrome Type II. Courtesy of Dr Jose Ganseman
-
WPW syndrome with an orthodromic circus movement tachycardia: Narrow complex tachycardia with a rate of 200 bpm (RR interval 320 ms). After 5 cycles, the tachycardia suddenly stops and four multiform complexes are seen without any P waves. These complexes should be regarded as a polymorphic ventricular tachycardia, which is not uncommon after an adenosine-terminated supraventricular tachycardia. A 5th complex is preceded by a P wave. The subsequent 4 complexes show a widened QRS complex and all are immediately preceded by a P wave. The initial phase of the QRS complex is slurred and positive in all available leads. Sinus rhythm continues thereafter with gradual abbreviation of the QRS complex until a 120 msec wide QRS complex remains.
-
The same patient's EKG during sinus rhythm. A discrete Δ wave is clearly visible. The morphology of the Δ wave suggests a left posterior Kent bundle.
Electrophysiologic Studies in WPW
The purpose of these studies is to:
- determine the location and number of accessory pathways.
- determine the mechanism and pathway of the tachycardia.
- to establish the diagnosis in those patients c a questionable resting EKG.
- to evaluate the efficacy of drugs.
- to select patients for surgery and to evaluate them postoperatively.
Risk stratification

Treatment is based on risk stratification of the individual. Risk stratification is performed to determine which individuals with WPW syndrome are at risk for sudden cardiac death (SCD). Sudden cardiac death in these individuals is due to the propagation of an atrial arrhythmia to the ventricles at a very high rate.
A good history should be taken to determine whether an individual has factors suggestive of a previous episode of unexplained syncope (fainting) or palpitations (sudden awareness of one's own, usually irregular, heartbeat). These may be due to earlier episodes of a tachycardia associated with the accessory pathway.
Individuals with WPW syndrome in whom the delta waves disappear with increases in the heart rate are considered at lower risk of SCD. This is because the loss of the delta wave shows that the accessory pathway cannot conduct electrical impulses at a high rate (in the anterograde direction). These individuals will typically not have fast conduction down the accessory pathway during episodes of atrial fibrillation.
Risk stratification is best performed via programmed electrical stimulation (PES) in the cardiac electrophysiology lab. This is an invasive procedure, in which the rate of impulse propagation via the accessory pathway is determined by stimulating the atria and by inducing transient atrial fibrillation.
High risk features that may be present during PES include an effective refractory period of the accessory pathway less than 270 ms, multiple pathways, septal location of pathway, and inducibility of supraventricular tachycardia. Individuals with any of these high risk features are generally considered at increased risk for SCD and should be treated accordingly.[8]
It is unclear whether invasive risk stratification (with programmed electrical stimulation) is necessary in the asymptomatic individual.[9] While some groups advocate PES for risk stratification in all individuals under 35 years old, others only offer it to individuals who have history suggestive of a tachyarrhythmia, since the incidence of sudden death is so low.[3][4]
Treatment
Acutely, people with WPW who are experiencing a tachydysrhythmia may require electrical cardioversion if their condition is critical, or, if more stable, medical treatment may be used. Patients with atrial fibrillation and rapid ventricular response are often treated with amiodarone or procainamide to stabilize their heart rate. Adenosine and other AV node blockers should be avoided in Atrial fibriliiatin with WPW; this inlcudes adenosine, diltiazem, verapamil,other calcium channel blockers and Beta-blockers. Patients with a rapid heart beat with narrow QRS complexes (circus movement tachycardias) may also be cardioverted, alternatively, adenosine may be administered if equipment for cardioversion is immediately available as a backup.
The definitive treatment of WPW syndrome is a destruction of the abnormal electrical pathway by radiofrequency catheter ablation. This procedure is performed almost exclusively by cardiac electrophysiologists. Radiofrequency catheter ablation is not performed in all individuals with WPW syndrome because there are inherent risks involved in the procedure. Adeosine is contraindicated for patients in atrial fibrillation or atrial flutter with a history of WPW
When performed by an experienced electrophysiologist, radiofrequency ablation has a high success rate.[10] If radiofrequency catheter ablation is successfully performed, the patient is generally considered cured. Recurrence rates are typically less than 5% after a successful ablation.[10] The one caveat is that individuals with underlying Ebstein's anomaly may develop additional accessory pathways during progression of their disease.
Circus Movement Tachycardias
- Rapid heart rate is most likely due to CMT or atrial fibrillation.
- Palpitations are usually regular during a CMT, irregularly with atrial fib.
- Some patients report that their attacks of CMT may be broken by vagal maneuvers.
- A 24 hr holter should be done in those patients with a suspicion of WPW to assess the mode of initiation, and to determine the type (CMT vs afib).
- If the patients quality of life is affected and the holter is negative, then EP studies should be done.
- If there is an inability to induce CMT at EP then it is very unlikely that this is the arrhythmia that the patient is experiencing outside the hospital. Inability to induce CMT obviously does not exclude that the patient is experiencing afib.
- If the patient presents in a rapid rhythm, you should first try vagal maneuvers to terminate a CMT.
- The most likely arrhythmia on a statistical basis is a CMT with the AP conducting in a retrograde fashion. If the AP is incorporated in a retrograde fashion then there are often retrograde P waves apparent following the QRS, with a PR interval longer than the RP'interval.
- If the patient is known to have WPW and has a regular tachycardia thought to be a CMT, then verapamil should be the first drug used. If verapamil is not available, then use propranolol IV. Both terminate the CMT by prolonging the refractory period at the AV node.
- Digitalis has been used, however some patients may dev afib with this, and dig may abbreviate the refractory period of the AP resulting in higher ventricular rates during the afib. Therefore the use of dig is not recommended.
- If drugs affecting the AV node are not effective, then drugs that affect the accessory pathway are used such as procainamide or disopyramide.
- If the above fail to terminate the tachycardia or if the patient is tolerating the tachycardia poorly, then the patient should be paced out of it or cardioverted. Pacing is safer in patients on multiple drugs.
Atrial Fibrillation
- Patients can experience high rates during afib because of conduction over the accessory pathway which can have a very short refractory period.
- Mean ventricular rates in these patients range from 160 to 300 BPM.
- During these attacks there is not only the risk of hemodynamic compromise but also a risk of degenerate into VF.
- As a rule dig should be avoided in these patients.
- Cardioversion is the tx of choice. If the patient is receiving drugs that promote asystole following electrical cardioversion (e.g. verapamil, beta-blockers, and probably amiodarone) then a temporary pacer should be positioned in the RV before the cardioversion.
- If the ventricular rate during the afib is not > 200, then you could try procainamide, disopyramide, or quinidine which may prolong the refractory period of the accessory pathway.
Drug Prophylaxis In The Patient With Proven Tachyarrhythmias
- Should receive prophylactic treatment if the arrhythmia is poorly tolerated.
- In Wellen's experience amiodarone is the most effective in preventing attacks of paroxysmal tachycardia in patients with WPW. Otherwise he recommends quinidine, disopyramide, procainamide or propranolol alone or in combination.
- For those patients with life-threatening rates during afib, Wellen's recommends amiodarone as prophylaxis which prolongs the AP refractory period. Quinidine is an alternative, but is less effective.
- These patients should undergo EP studies to assess the adequacy of treatment.
- These authors state that the refractory period of the AP can shorten in the presence of sympathetic stimulation and advocate the addition of a beta blocker.
Approach to the Patient with a Questionable EKG
- As mentioned previously, the diagnosis can be difficult in those patients c normal EKGs at rest.
- In some patients the diagnosis can be made with the following noninvasive procedures:
- CSP to increase the AV nodal delay therefore enhancing conduction over the accessory pathway.
- IV procainamide may cause QRS abnormality to disappear by prolonging conduction down the AP.
- β-blockers, verapamil, digoxin may also facilitate the conduction down the accessory pathway
Differential Diagnosis
Diagnosis of Hypertrophy, Bundle Branch Block and MI in the Presence of WPW are all obscured.
- Type A WPW
- RBBB
- RVH
- True posterior MI
- IMI
- Type B WPW
- LBBB
- Anterior MI
- IMI
- WPW and atrial flutter
- paroxysmal VT or flutter
Intractable Tachyarrhythmias in WPW
In patients with afib with rapid ventricular response then surgical interruption should be considered.
Variants of WPW
- LGL: Lown-Ganong-Levine Syndrome
- there is a short PR, but no delta wave
- due to intranodal bypass tracts (i.e. there is conduction down James fibers)
- normal QRS duration
- PR less than 0.12 seconds
- normal P wave
- Mahaim Type of Preexcitation
- nodoventricular, nodofascicular or fasciculoventricular connections
- the impulse may travel through the AV node normally and this may then be followed by premature conduction to the basal ventricular myocardium
- there is a delta wave with a normal PR interval
- is rarer than WPW or LGL
- in older patients there can be a prolonged conduction down the accessory pathway resulting in a normal PR interval in the presence of WPW which is tough to distinguish from Mahaim fibers
-
Figure 1: A 24 years old man with Mahaim type of preexcitation
-
Figure 2: The same patient after Mahaim bundle ablation
References
- ↑ Rosner MH, Brady WJ Jr, Kefer MP, Martin ML. (1999). "Electrocardiography in the patient with the Wolff-Parkinson-White syndrome: diagnostic and initial therapeutic issues". American Journal of Emergency Medicine. 17 (7): 705–14. PMID 10597097.
- ↑ Sorbo MD, Buja GF, Miorelli M, Nistri S, Perrone C, Manca S, Grasso F, Giordano GM, Nava A. (1995). "The prevalence of the Wolff-Parkinson-White syndrome in a population of 116,542 young males". Giornale Italiano di Cardiologia (in Italian). 25 (6): 681–7. PMID 7649416.
- ↑ 3.0 3.1 3.2 Munger TM, Packer DL, Hammill SC, Feldman BJ, Bailey KR, Ballard DJ, Holmes DR Jr, Gersh BJ. (1993). "A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989". Circulation. 87 (3): 866–73. PMID 8443907.
- ↑ 4.0 4.1 Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB (2001). "The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years". American Heart Journal. 142 (3): 530–6. PMID 11526369 doi:10.1067/mhj.2001.117779.
- ↑ Template:WhoNamedIt
- ↑ L. Wolff, J. Parkinson, P. D. White. Bundle-branch block with short P-R interval in healthy young people prone to paroxysmal tachyardia. American Heart Journal, St. Louis, 1930, 5: 685.
- ↑ Mashima Y, Kigasawa K, Hasegawa H, Tani M, Oguchi Y. (1996). "High incidence of pre-excitation syndrome in Japanese families with Leber's hereditary optic neuropathy" (subscription required). Clinical Genetics. 50 (6): 535–7. PMID 9147893.
- ↑ Pappone C, Santinelli V, Manguso F, Augello G, Santinelli O, Vicedomini G, Gulletta S, Mazzone P, Tortoriello V, Pappone A, Dicandia C, Rosanio S. (2003). "A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome" (free registration required). New England Journal of Medicine. 349 (19): 1803–11. PMID 14602878.
- ↑ Campbell RM, Strieper MJ, Frias PA, Collins KK, Van Hare GF, Dubin AM (2003). "Survey of current practice of pediatric electrophysiologists for asymptomatic Wolff-Parkinson-White syndrome". Pediatrics. 111 (3): e245–7. PMID 12612279.
- ↑ 10.0 10.1 Pappone C, Lamberti F, Santomauro M, Stabile G, De Simone A, Turco P, Pannain S, Loricchio ML, Rotunno R, Chiariello M (1993). "Ablation of paroxysmal tachycardia in Wolff-Parkinson-White syndrome". Cardiologia (in Italian). 38 (12 Suppl 1): 189–97. PMID 8020017.
Additional Resources
- Adapted from The Wolff-Parkinson-White Syndrome, Wellens et al, Cardiac Arrhythmias, Mandel, Second Edition, Lippincott.
See also
- Lown-Ganong-Levine syndrome
- Cardiac electrophysiology
- Electrical conduction system of the heart
- Electrocardiogram
- Electrophysiologic study
External links
- Wolff-Parkinson-White syndrome information from Seattle Children's Hospital Heart Center
de:Wolff-Parkinson-White-Syndrom it:Sindrome di Wolff-Parkinson-White