Vigabatrin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 16: | Line 16: | ||
Respiratory: Bronchitis (infantile spasms, 30% ), Upper respiratory infection (complex partial seizures, 7% to 10%; infantile spasms, 46% to 51% ) | Respiratory: Bronchitis (infantile spasms, 30% ), Upper respiratory infection (complex partial seizures, 7% to 10%; infantile spasms, 46% to 51% ) | ||
Other: Fatigue (9% to 28% ) | Other: Fatigue (9% to 28% ) | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;"> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">VISION LOSS</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> ( | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> | ||
*SABRIL causes permanent bilateral concentric visual field constriction. Because assessing vision may be difficult in infants and children, the frequency and extent of vision loss is poorly characterized in these patients. For this reason, the risk described below is primarily based on the adult experience. | |||
*Based upon adult studies, 30 percent or more of patients can be affected, ranging in severity from mild to severe, including tunnel vision to within 10 degrees of visual fixation, and can result in disability. In some cases, SABRIL also can damage the central retina and may decrease visual acuity. | |||
*The onset of vision loss from SABRIL is unpredictable, and can occur within weeks of starting treatment or sooner, or at any time after starting treatment, even after months or years. | |||
*Symptoms of vision loss from SABRIL are unlikely to be recognized by patients or caregivers before vision loss is severe. Vision loss of milder severity, while often unrecognized by the patient or caregiver, can still adversely affect function. | |||
*The risk of vision loss increases with increasing dose and cumulative exposure, but there is no dose or exposure known to be free of risk of vision loss. | |||
*Unless a patient is formally exempted from periodic ophthalmologic assessment as documented in the SHARE program, vision should be assessed to the extent possible at baseline (no later than 4 weeks after starting SABRIL) and at least every 3 months during therapy. Vision assessment is also required about 3 to 6 months after the discontinuation of SABRIL therapy. | |||
*Once detected, vision loss due to SABRIL is not reversible. It is expected that, even with frequent monitoring, some patients will develop severe vision loss. | |||
*Drug discontinuation should be considered, balancing benefit and risk, if visual loss is documented. | |||
It is possible that vision loss can worsen despite discontinuation of SABRIL. | |||
*Because of the risk of visual loss, SABRIL should be withdrawn from patients with refractory complex partial seizures who fail to show substantial clinical benefit within 3 months of initiation and within 2-4 weeks of initiation for patients with infantile spasms, or sooner if treatment failure becomes obvious. Patient response to and continued need for SABRIL should be periodically reassessed. | |||
*SABRIL should not be used in patients with, or at high risk of, other types of irreversible vision loss unless the benefits of treatment clearly outweigh the risks. The interaction of other types of irreversible vision damage with vision damage from SABRIL has not been well-characterized, but is likely adverse. | |||
*SABRIL should not be used with other drugs associated with serious adverse ophthalmic effects such as retinopathy or glaucoma unless the benefits clearly outweigh the risks. | |||
*The possibility that vision loss from SABRIL may be more common, more severe or have more severe functional consequences in infants and children than in adults cannot be excluded. | |||
*The lowest dose and shortest exposure to SABRIL consistent with clinical objectives should be used. | |||
Because of the risk of permanent vision loss, SABRIL is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the SHARE PrograM. Further information is available at [www.sabril.net or 1-888-45-SHARE]. | |||
|fdaLIADAdult=====Important Dosing Instructions==== | |||
*SABRIL is given orally with or without food. The SABRIL dosing regimen depends on the indication, age group, weight, and dosage form (tablets or powder for oral solution). Patients with impaired renal function require dose adjustment. | |||
*SABRIL tablets and powder for oral solution are bioequivalent. Either tablet or powder can be used for CPS. Powder for oral solution should be used for IS; tablets should not be used for IS because of difficulty in the administration of tablets to infants and young children. | |||
*SABRIL powder for oral solution should be mixed with water prior to administration. | |||
If using SABRIL powder for oral solution, physicians should review and discuss the Medication Guide and instructions for mixing and giving SABRIL with the patient or caregiver(s). Physicians should confirm that patients or caregiver(s) understand how to mix SABRIL powder with water and administer the correct daily dose. Empty the entire contents of each 500 mg packet into a clean cup, and dissolve in 10 mL of cold or room temperature water per packet (see Table 2). Administer the resulting solution using the 10 mL oral syringe supplied with the medication. The concentration of the final solution is 50 mg/mL. Discard the resulting solution if it is not clear (or free of particles) and colorless. Each individual dose should be prepared and used immediately. Discard any unused portion of the solution after administering the correct dose. Monitoring of SABRIL plasma concentrations to optimize therapy is not helpful. If a decision is made to discontinue SABRIL, the dose should be gradually reduced | |||
====Refractory Complex Partial Seizures==== | |||
======Adults (Patients >16 Years of Age)====== | |||
Treatment should be initiated at 1000 mg/day (500 mg twice daily). Total daily dose may be increased in 500 mg increments at weekly intervals depending on response. The recommended dose of SABRIL in adults is 3000 mg/day (1500 mg twice daily). A 6000 mg/day dose has not been shown to confer additional benefit compared to the 3000 mg/day dose and is associated with an increased incidence of adverse events. In controlled clinical studies in adults with complex partial seizures, SABRIL was tapered by decreasing the daily dose 1000 mg/day on a weekly basis until discontinued. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Vigabatrin in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Vigabatrin in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Vigabatrin in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Vigabatrin in adult patients. | ||
|fdaLIADPed=====Refractory Complex Partial Seizures==== | |||
======Adults (Patients >16 Years of Age)====== | |||
Treatment is based on body weight as shown in Table 1. Treatment should be initiated at a total daily dose of 500 mg/day (250 mg twice daily) and may be increased weekly to a total maintenance dose of 2000 mg/day (1000 mg twice daily). Patients weighing more than 60 kg should be dosed according to adult recommendations. | |||
[[file:Pediaric Dosing information.png|none|350px]] | |||
====Infantile Spasms==== | |||
The initial daily dosing is 50 mg/kg/day given in two divided doses; subsequent dosing can be titrated by 25 mg/kg/day to 50 mg/kg/day increments every 3 days up to a maximum of 150 mg/kg/day given in 2 divided doses [see USE IN SPECIFIC POPULATIONS (8.4)]. | |||
Table 2 below describes how many packets and how many milliliters (mL) of water will be needed to prepare each individual dose. The concentration after reconstitution is 50 mg/mL. | |||
[[file:Vigabatrin IS dosage.png|none|350px]] | |||
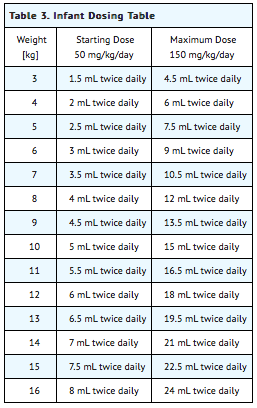
Table 3 provides the volume of the 50 mg/mL dosing solution that should be administered as individual doses in infants of various weights. | |||
[[file:Vigabatrin IS DOsage2.png|none|350px]] | |||
In a controlled clinical study in patients with infantile spasms, SABRIL was tapered by decreasing the daily dose at a rate of 25 mg/kg to 50 mg/kg every 3 to 4 days | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Vigabatrin in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Vigabatrin in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Vigabatrin in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Vigabatrin in pediatric patients. | ||
Revision as of 15:58, 23 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
VISION LOSS
See full prescribing information for complete Boxed Warning.
Condition Name:
It is possible that vision loss can worsen despite discontinuation of SABRIL.
|
Overview
Vigabatrin is a anticonvulsant, gamma aminobutyric acid transaminase inhibitor that is FDA approved for the treatment of refractory complex partial seizures and infantile spams. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Weight increased (6% to 47% ) Musculoskeletal: Arthralgia (complex partial seizures, 10% ) Neurologic: Confusion (complex partial seizures, 4% ), Coordination problem (complex partial seizures, 7% ), Memory impairment (complex partial seizures, 7% ), Somnolence (6% to 45% ), Tremor (complex partial seizures, 6% to 15% ) Ophthalmic: Blurred vision (complex partial seizures, 13% ), Diplopia (complex partial seizures, 5% to 7% ), Nystagmus (complex partial seizures, 5% to 13% ) Otic: Infection of ear (infantile spasms, 7% to 14% ), Otitis media (complex partial seizures, 6%; infantile spasms, 10% to 44% ) Psychiatric: Aggressive behavior (complex partial seizures, 5% ) Reproductive: Dysmenorrhea (complex partial seizures, 9% ) Respiratory: Bronchitis (infantile spasms, 30% ), Upper respiratory infection (complex partial seizures, 7% to 10%; infantile spasms, 46% to 51% ) Other: Fatigue (9% to 28% ).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Important Dosing Instructions
- SABRIL is given orally with or without food. The SABRIL dosing regimen depends on the indication, age group, weight, and dosage form (tablets or powder for oral solution). Patients with impaired renal function require dose adjustment.
- SABRIL tablets and powder for oral solution are bioequivalent. Either tablet or powder can be used for CPS. Powder for oral solution should be used for IS; tablets should not be used for IS because of difficulty in the administration of tablets to infants and young children.
- SABRIL powder for oral solution should be mixed with water prior to administration.
If using SABRIL powder for oral solution, physicians should review and discuss the Medication Guide and instructions for mixing and giving SABRIL with the patient or caregiver(s). Physicians should confirm that patients or caregiver(s) understand how to mix SABRIL powder with water and administer the correct daily dose. Empty the entire contents of each 500 mg packet into a clean cup, and dissolve in 10 mL of cold or room temperature water per packet (see Table 2). Administer the resulting solution using the 10 mL oral syringe supplied with the medication. The concentration of the final solution is 50 mg/mL. Discard the resulting solution if it is not clear (or free of particles) and colorless. Each individual dose should be prepared and used immediately. Discard any unused portion of the solution after administering the correct dose. Monitoring of SABRIL plasma concentrations to optimize therapy is not helpful. If a decision is made to discontinue SABRIL, the dose should be gradually reduced
Refractory Complex Partial Seizures
Adults (Patients >16 Years of Age)
Treatment should be initiated at 1000 mg/day (500 mg twice daily). Total daily dose may be increased in 500 mg increments at weekly intervals depending on response. The recommended dose of SABRIL in adults is 3000 mg/day (1500 mg twice daily). A 6000 mg/day dose has not been shown to confer additional benefit compared to the 3000 mg/day dose and is associated with an increased incidence of adverse events. In controlled clinical studies in adults with complex partial seizures, SABRIL was tapered by decreasing the daily dose 1000 mg/day on a weekly basis until discontinued.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vigabatrin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vigabatrin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Refractory Complex Partial Seizures
Adults (Patients >16 Years of Age)
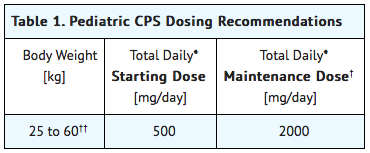
Treatment is based on body weight as shown in Table 1. Treatment should be initiated at a total daily dose of 500 mg/day (250 mg twice daily) and may be increased weekly to a total maintenance dose of 2000 mg/day (1000 mg twice daily). Patients weighing more than 60 kg should be dosed according to adult recommendations.
Infantile Spasms
The initial daily dosing is 50 mg/kg/day given in two divided doses; subsequent dosing can be titrated by 25 mg/kg/day to 50 mg/kg/day increments every 3 days up to a maximum of 150 mg/kg/day given in 2 divided doses [see USE IN SPECIFIC POPULATIONS (8.4)].
Table 2 below describes how many packets and how many milliliters (mL) of water will be needed to prepare each individual dose. The concentration after reconstitution is 50 mg/mL.

Table 3 provides the volume of the 50 mg/mL dosing solution that should be administered as individual doses in infants of various weights.
In a controlled clinical study in patients with infantile spasms, SABRIL was tapered by decreasing the daily dose at a rate of 25 mg/kg to 50 mg/kg every 3 to 4 days
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vigabatrin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vigabatrin in pediatric patients.
Contraindications
There is limited information regarding Vigabatrin Contraindications in the drug label.
Warnings
|
VISION LOSS
See full prescribing information for complete Boxed Warning.
Condition Name:
It is possible that vision loss can worsen despite discontinuation of SABRIL.
|
There is limited information regarding Vigabatrin Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Vigabatrin Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Vigabatrin Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Vigabatrin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Vigabatrin in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Vigabatrin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Vigabatrin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Vigabatrin in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Vigabatrin in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Vigabatrin in geriatric settings.
Gender
There is no FDA guidance on the use of Vigabatrin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Vigabatrin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Vigabatrin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Vigabatrin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Vigabatrin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Vigabatrin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Vigabatrin Administration in the drug label.
Monitoring
There is limited information regarding Vigabatrin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Vigabatrin and IV administrations.
Overdosage
There is limited information regarding Vigabatrin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Vigabatrin Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Vigabatrin Mechanism of Action in the drug label.
Structure
There is limited information regarding Vigabatrin Structure in the drug label.
Pharmacodynamics
There is limited information regarding Vigabatrin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Vigabatrin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Vigabatrin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Vigabatrin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Vigabatrin How Supplied in the drug label.
Storage
There is limited information regarding Vigabatrin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Vigabatrin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Vigabatrin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Vigabatrin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Vigabatrin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Vigabatrin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Vigabatrin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| File:Vigabatrin.png | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80-90% |
| Protein binding | 0 |
| Metabolism | Almost no metabolic transformation occurs |
| Elimination half-life | 5-8 hours in young adults, 12-13 hours in the elderly. |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C6H11NO2 |
| Molar mass | 129.157 g/mol |
Vigabatrin is an anticonvulsant that inhibits the catabolism of GABA. It is an analog of GABA, but it is not a receptor agonist.[1]
Mechanism of action
Vigabatrin is an irreversible inhibitor of gamma-aminobutyric acid transaminase (GABA-T), the enzyme responsible for the catabolism of GABA, which increases the level of GABA in the synapses.[1]
Vigabatrin is a racemic compound, and its [S]-enantiomer is pharmacologically active.[2],[3]
Pharmacokinetics
With most drugs, elimination half-life is a useful predictor of dosing schedules and the time needed to reach steady state concentrations. In the case of vigabatrin, however, it has been found that the half-life of biologic activity is far longer than the elimination half-life.[4]
For vigabatrin, there is no range of target concentrations because researchers found no difference between the serum concentration levels of responders and those of non-responders.[5] Instead, the duration of action is believed to be more a function of the GABA-T resynthesis rate; levels of GABA-T do not usually return to their normal state until six days after stopping the medication.[3]
Uses
Approved/clinically proven
Canada
In Canada, vigabatrin is approved for use as an adjunctive treatment (with other drugs) in treatment resistant epilepsy, complex partial seizures, secondary generalized seizures, and for monotherapy use in infantile spasms in West syndrome.[1]
Mexico
As of 2003, vigabatrin is approved in Mexico for the treatment of epilepsy that is not satisfactorily controlled by conventional therapy (adjunctive or monotherapy) or in recently diagnosed patients who have not tried other agents (monotherapy).[6]
Vigabatrin is also indicated for monotherapy use in secondarily generalized tonic-clonic seizures, partial seizures, and in infantile spasms due to West syndrome.[6]
Unapproved/Investigational
In November of 2001, a team of scientists lead by Peter Zwanzger of the University of Munich reported that vigabatrin reduced cholecystokinin tetrapeptide-induced symptoms of panic disorder, in addition to elevated cortisol and ACTH levels, in healthy volunteers.[7]
In 1994, Feucht and Brantner-Inthaler reported that vigabatrin reduced seizures by 50-100% in 85% of children with Lennox-Gastaut syndrome who had poor results with a valproate.[8]
In 1984, a double-blind crossover-study of six Huntington's disease patients—five of them on antipsychotics—reported that vigabatrin did little, if anything, to improve hyperkinetic movements, the ability to carry out daily activities, or normalize motor function.[9]
Adverse effects
Central nervous system
Common
Out of 2,081 subjects, somnolence (12.5%), headache (3.8%), dizziness (3.8%), nervousness (2.7%), depression (2.5%), memory disturbances (2.3%), diplopia (2.2%), aggression (2.0%), ataxia (1.9%), vertigo (1.9%), hyperactivity (1.8%), vision abnormalities (1.6%), confusion (1.4%), insomnia (1.3%), impaired concentration (1.2%), personality disorder (1.1%).[1] Out of 299 children, 33 (11%) became hyperactive.[1]
Rare
Some patients develop psychosis during the course of vigabatrin therapy,[10] which is more common in adults than in children.[11] This can happen even in patients with no prior history of psychosis.[12] Other rare CNS side effects include anxiety, emotional lability, irritability, tremor, abnormal gait, and speech disorder.[1]
Gastrointestinal
Common
Abdominal pain (1.6%), constipation (1.4%), vomiting (1.4%), and nausea (1.4%).[1]
Rare
Dyspepsia and increased appetite occurred in less than 1% of subjects in clinical trials.[1]
Body as a Whole
Common
Fatigue (9.2%), weight gain (5.0%), asthenia (1.1%).[1]
Teratogenicity
A teratology study conducted in rabbits found that a dose of 150mg/kg/day caused cleft palate in 2% of pups and a dose of 200 mg/kg/day caused it in 9%.[1] This may be due to a decrease in methionine levels, according to a study published in March of 2001.[13] In 2005, a study conducted at the University of Catania was published stating that rats whose mothers had consumed 250-1000 mg/kg/day had poorer performance in the water maze and open-field tasks, rats in the 750-mg group were underweight at birth and did not catch up to the control group, and rats in the 1000 mg group did not survive pregnancy.[14]
There is no controlled teratology data in humans to date.
More on "abnormal vision"
In 2003, vigabatrin was shown by Frisén and Malmgren to cause irreversible diffuse atrophy of the retinal nerve fiber layer in a retrospective study of 25 patients.[15] This has the most effect on the outer area (as opposed to the macular, or central area) of the retina.[16]
Drug interactions
A study published in 2002 found that vigabatrin causes a statistically significant increase in plasma clearance of carbamazepine.[17]
In 1984, Drs Rimmer and Richens at the University of Wales reported that administering vigabatrin with phenytoin lowered the serum phenytoin concentration in patients with treatment-resistant epilepsy.[18] The concentration of phenytoin falls to 23% within five weeks, according to an experiment published in 1989 by the same two scientists that tried and failed to elucidate the mechanism behind this interaction.[19]
Brand names
Vigabatrin is sold as Sabril® in Canada,[20] Mexico,[6] and the United Kingdom.[21] The brand name in Denmark is Sabrilex®.
References and end notes
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Long, Phillip W. "Vigabatrin." Internet Mental Health. 1995-2003.
- ↑ Sheean, G. (1992). "Vigabatrin--plasma enantiomer concentrations and clinical effects". Clinical and Experimental Neurology. 29: 107–16. PMID 1343855. Unknown parameter
|coauthors=ignored (help) - ↑ 3.0 3.1 Gram L, Larsson OM, Johnsen A, Schousboe A (1989). "Experimental studies of the influence of vigabatrin on the GABA system". British Journal of Clinical Pharmacology. 27 (Suppl 1): 13S–17S. PMID 2757904.
- ↑ Browne TR (1998). "Pharmacokinetics of antiepileptic drugs". Neurology. 51 (5 suppl 4): S2–7. PMID 9818917.
- ↑ Lindberger M, Luhr O, Johannessen SI, Larsson S, Tomson T (2003). "Serum concentrations and effects of gabapentin and vigabatrin: observations from a dose titration study". Therapeutic Drug Monitoring. 25 (4): 457–62. PMID 12883229.
- ↑ 6.0 6.1 6.2 DEF MEXICO: SABRIL Diccionario de Especialdades Farmaceuticas. Edicion 49, 2003.
- ↑ Zwanzger P, Baghai TC, Schuele C, Strohle A, Padberg F, Kathmann N, Schwarz M, Moller HJ, Rupprecht R (2001). "Vigabatrin decreases cholecystokinin-tetrapeptide (CCK-4) induced panic in healthy volunteers". Neuropsychopharmacology. 25 (5): 699–703. PMID 11682253.
- ↑ Feucht M, Brantner-Inthaler S (1994). "Gamma-vinyl-GABA (vigabatrin) in the therapy of Lennox-Gastaut syndrome: an open study" (PDF). Epilepsia. 35 (5): 993–8. PMID 7925171. Retrieved 2006-05-25.
- ↑ Scigliano G, Giovannini P, Girotti F, Grassi MP, Caraceni T, Schechter PJ (1984). "Gamma-vinyl GABA treatment of Huntington's disease". Neurology. 34 (1): 94–6. PMID 6228746.
- ↑ Sander JW, Hart YM (1990). "Vigabatrin and behaviour disturbance". Lancet. 335 (8680): 57. PMID 1967367.
- ↑ Chiaretti A, Castorina M, Tortorolo L, Piastra M, Polidori G (1994). "[Acute psychosis and vigabatrin in childhood]". La Pediatria Medica e Chirurgica : Medical and surgical pediatrics. 16 (5): 489–90. [Article in Italian] PMID 7885961
- ↑ Sander JW, Hart YM, Trimble MR, Shorvon SD (1991). "Vigabatrin and psychosis". Journal of Neurology, Neurosurgery, and Psychiatry. 54 (5): 435–9. PMID 1865207.
- ↑ Abdulrazzaq YM, Padmanabhan R, Bastaki SM, Ibrahim A, Bener A (2001). "Placental transfer of vigabatrin (gamma-vinyl GABA) and its effect on concentration of amino acids in the embryo of TO mice". Teratology. 63 (3): 127–33. PMID 11283969.
- ↑ Lombardo SA, Leanza G, Meli C, Lombardo ME, Mazzone L, Vincenti I, Cioni M (2005). "Maternal exposure to the antiepileptic drug vigabatrin affects postnatal development in the rat". Neurological Sciences. 26 (2): 89–94. PMID 15995825.
- ↑ Frisén L, Malmgren K (2003). "Characterization of vigabatrin-associated optic atrophy". Acta Ophthalmologica Scandinavica. 81 (5): 466–73. PMID 14510793.
- ↑ Buncic JR, Westall CA, Panton CM, Munn JR, MacKeen LD, Logan WJ (2004). "Characteristic retinal atrophy with secondary "inverse" optic atrophy identifies vigabatrin toxicity in children". Ophthalmology. 111 (10): 1935–42. PMID 15465561.
- ↑ Sanchez-Alcaraz, Agustín (2002). "Effect of vigabatrin on the pharmacokinetics of carbamazepine". Journal of Clinical Pharmacology and Therapeutics. 27 (6): 427–30. PMID 12472982. Unknown parameter
|coauthors=ignored (help) - ↑ Rimmer EM, Richens A (1984). "Double-blind study of gamma-vinyl GABA in patients with refractory epilepsy". Lancet. 1 (8370): 189–90. PMID 6141335.
- ↑ Rimmer EM, Richens A (1989). "Interaction between vigabatrin and phenytoin". British Journal of Clinical Pharmacology. 27 (Suppl 1): 27S–33S. PMID 2757906.
- ↑ drugs.com Vigabatrin Drug Information
- ↑ Treatments for Epilepsy - Vigabatrin Norfolk and Waveney Mental Health Partnership NHS Trust
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- Pages with broken file links
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Anticonvulsants