Tricuspid stenosis surgery: Difference between revisions
No edit summary |
|||
| (8 intermediate revisions by one other user not shown) | |||
| Line 7: | Line 7: | ||
==Surgery== | ==Surgery== | ||
{| style="cellpadding=0; cellspacing= 0; width: 800px;" | |||
|- | |||
| style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF;" align=center |'''Recommendations for intervention in tricuspid valve disease''' | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #B8B8B8" align=left | ''' Tricuspid stenosis ([[ ESC guidelines classification scheme|Class I, Level of Evidence C]]):''' | |||
|- | |||
|style="padding: 0 5px; font-size: 100%; background: #F5F5F5; width: 70%" align=left| | |||
❑ [[Surgery]] is recommended in symptomatic [[patients]] with severe [[tricuspid stenosis]]<br> | |||
❑ [[Surgery]] is recommended in patients with severe [[tricuspid stenosis]] undergoing left-sided [[valve]] [[intervention]]<br> | |||
| | |||
|} | |||
{| | |||
! colspan="2" style="background: PapayaWhip;" align="center" + |The above table adopted from 2021 ESC Guideline<ref name="pmid34453165">{{cite journal |vauthors=Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W |title=2021 ESC/EACTS Guidelines for the management of valvular heart disease |journal=Eur Heart J |volume=43 |issue=7 |pages=561–632 |date=February 2022 |pmid=34453165 |doi=10.1093/eurheartj/ehab395 |url=}}</ref> | |||
|- | |||
|} | |||
*[[Surgery]] is the mainstay of treatment for [[tricuspid stenosis]] (TS), which includes the following:<ref name="pmid24589852">{{cite journal| author=Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA et al.| title=2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. | journal=Circulation | year= 2014 | volume= | issue= | pages= | pmid=24589852 | doi=10.1161/CIR.0000000000000029 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24589852 }} </ref><ref name="YunokiNaruko2006">{{cite journal|last1=Yunoki|first1=Kei|last2=Naruko|first2=Takahiko|last3=Itoh|first3=Akira|last4=Ohashi|first4=Junko|last5=Fujimoto|first5=Kohei|last6=Shirai|first6=Naoya|last7=Shimamura|first7=Koichi|last8=Komatsu|first8=Ryushi|last9=Sakanoue|first9=Yuji|last10=Haze|first10=Kazuo|title=Percutaneous Transcatheter Balloon Valvuloplasty for Bioprosthetic Tricuspid Valve Stenosis|journal=Circulation|volume=114|issue=18|year=2006|issn=0009-7322|doi=10.1161/CIRCULATIONAHA.106.618611}}</ref> | *[[Surgery]] is the mainstay of treatment for [[tricuspid stenosis]] (TS), which includes the following:<ref name="pmid24589852">{{cite journal| author=Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA et al.| title=2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. | journal=Circulation | year= 2014 | volume= | issue= | pages= | pmid=24589852 | doi=10.1161/CIR.0000000000000029 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24589852 }} </ref><ref name="YunokiNaruko2006">{{cite journal|last1=Yunoki|first1=Kei|last2=Naruko|first2=Takahiko|last3=Itoh|first3=Akira|last4=Ohashi|first4=Junko|last5=Fujimoto|first5=Kohei|last6=Shirai|first6=Naoya|last7=Shimamura|first7=Koichi|last8=Komatsu|first8=Ryushi|last9=Sakanoue|first9=Yuji|last10=Haze|first10=Kazuo|title=Percutaneous Transcatheter Balloon Valvuloplasty for Bioprosthetic Tricuspid Valve Stenosis|journal=Circulation|volume=114|issue=18|year=2006|issn=0009-7322|doi=10.1161/CIRCULATIONAHA.106.618611}}</ref> | ||
| Line 32: | Line 47: | ||
* In patients with [[carcinoid syndrome]], using a [[mechanical valve]] over a [[bioprosthetic valve]] is better choice to avoid the [[degeneration]] on the valve.<br /> | * In patients with [[carcinoid syndrome]], using a [[mechanical valve]] over a [[bioprosthetic valve]] is better choice to avoid the [[degeneration]] on the valve.<br /> | ||
== | == 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines<ref name="pmid33332150">{{cite journal| author=Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F | display-authors=etal| title=2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. | journal=Circulation | year= 2021 | volume= 143 | issue= 5 | pages= e72-e227 | pmid=33332150 | doi=10.1161/CIR.0000000000000923 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=33332150 }}</ref> == | ||
{| class="wikitable" style="width: 80%;" | |||
{|class="wikitable" | |- | ||
| colspan="1" style="text-align:center; background:LightGreen" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class I]] | |||
|- | |- | ||
| | | bgcolor="LightGreen" |1. In patients with severe TR (Stages C and D) undergoing left-sided valve surgery, tricuspid valve surgery is recommended ''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | ||
|} | |||
{| class="wikitable" style="width: 80%;" | |||
|- | |- | ||
| | | colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIa]] | ||
|- | |- | ||
| bgcolor=" | | bgcolor="LemonChiffon" |2. In patients with progressive TR (Stage B) undergoing left-sided valve surgery, tricuspid valve surgery can be beneficial in the context of either 1) tricuspid annular dilation (tricuspid annulus end diastolic diameter >4.0 cm) or 2) prior signs and symptoms of right-sided HF.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | ||
3. In patients with signs and symptoms of right-sided HF and severe primary TR (Stage D), isolated tricuspid valve surgery can be beneficial to reduce symptoms and recurrent hospitalizations.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
4. In patients with signs and symptoms of right-sided HF and severe isolated secondary TR attributable to annular dilation (in the absence of pulmonary hypertension or left-sided disease) who are poorly responsive to medical therapy (Stage D), isolated tricuspid valve surgery can be beneficial to reduce symptoms and recurrent hospitalizations.''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |} | ||
{| class="wikitable" style="width: 80%;" | |||
{|class="wikitable | |||
|- | |- | ||
| | | colspan="1" style="text-align:center; background:LemonChiffon" |[[ACC AHA guidelines classification scheme#Classification of Recommendations|Class IIb]] | ||
|- | |- | ||
| bgcolor="LemonChiffon" |5. In asymptomatic patients with severe primary TR (Stage C) and progressive RV dilation or systolic dysfunction, isolated tricuspid valve surgery may be considered''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: C-LD)]]'' | |||
6. In patients with signs and symptoms of right-sided HF and severe TR (Stage D) who have undergone previous left-sided valve surgery, reoperation with isolated tricuspid valve surgery may be considered in the absence of severe pulmonary hypertension or severe RV systolic dysfunction''([[ACC AHA guidelines classification scheme#Level of Evidence|Level of Evidence: B-NR)]]'' | |||
|} | |} | ||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
Latest revision as of 14:17, 8 December 2022
|
Tricuspid stenosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tricuspid stenosis surgery On the Web |
|
American Roentgen Ray Society Images of Tricuspid stenosis surgery |
|
Risk calculators and risk factors for Tricuspid stenosis surgery |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vamsikrishna Gunnam M.B.B.S [2] ;Mohammed Salih, M.D.; Syed Musadiq Ali M.B.B.S.[3] Rim Halaby, M.D. [4]
Overview
Surgical tricuspid valve replacement in tricuspid stenosis (TS) is recommended among patients undergoing surgical intervention for left valvular disease as well as among patients with severe symptomatic isolated tricuspid stenosis (TS).
Surgery
| Recommendations for intervention in tricuspid valve disease | |
| Tricuspid stenosis (Class I, Level of Evidence C): | |
|
❑ Surgery is recommended in symptomatic patients with severe tricuspid stenosis |
| The above table adopted from 2021 ESC Guideline[1] |
|---|
- Surgery is the mainstay of treatment for tricuspid stenosis (TS), which includes the following:[2][3]
Valvotomy
- Valvotomy in tricuspid stenosis (TS) patients is done by using 1, 2, or 3 balloons.[4][5][6][7][8]
- By undergoing balloon valvotomy the tricuspid valve area increase from less than 1 to almost 2 cm2.
- In cases of severe rheumatic tricuspid stenosis using a inoue balloon is much more useful and a simplified approach.[9]
- With valvotomy there is significant change in transvalvular pressure gradient across tricuspid valve and there is a decrease in right atrial pressure.
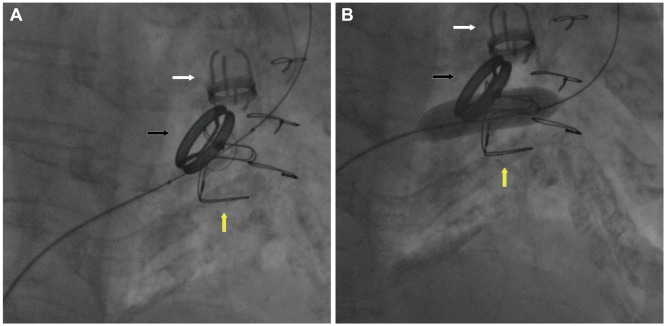
Valve surgery
- In tricuspid stenosis (TS) patients valve surgery include either valve repair or valve replacement.[11][12][13]
- Consider repair of the tricuspid valve if its feasible.
- If tricuspid valve repair not an option consider valve replacement.
- Patients who are undergoing tricuspid valve replacement the mortality rate is little higher when compared to patients who are undergoing tricuspid valve repair.
- While considering tricuspid valve replacement it can be done in 2 ways:
- Open tricuspid valve replacement
- Transcatheter replacement
- Surgeon can choose either a bioprosthetic valve or an mechanical valve, the outcome is same using either of the valves with some conditions as an exception.
- Survival in patients with bioprosthetic valve or an mechanical valve is almost the same.
- In patients with carcinoid syndrome, using a mechanical valve over a bioprosthetic valve is better choice to avoid the degeneration on the valve.
2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines[14]
| Class I |
| 1. In patients with severe TR (Stages C and D) undergoing left-sided valve surgery, tricuspid valve surgery is recommended (Level of Evidence: B-NR) |
| Class IIa |
| 2. In patients with progressive TR (Stage B) undergoing left-sided valve surgery, tricuspid valve surgery can be beneficial in the context of either 1) tricuspid annular dilation (tricuspid annulus end diastolic diameter >4.0 cm) or 2) prior signs and symptoms of right-sided HF.(Level of Evidence: B-NR)
3. In patients with signs and symptoms of right-sided HF and severe primary TR (Stage D), isolated tricuspid valve surgery can be beneficial to reduce symptoms and recurrent hospitalizations.(Level of Evidence: B-NR) 4. In patients with signs and symptoms of right-sided HF and severe isolated secondary TR attributable to annular dilation (in the absence of pulmonary hypertension or left-sided disease) who are poorly responsive to medical therapy (Stage D), isolated tricuspid valve surgery can be beneficial to reduce symptoms and recurrent hospitalizations.(Level of Evidence: B-NR) |
| Class IIb |
| 5. In asymptomatic patients with severe primary TR (Stage C) and progressive RV dilation or systolic dysfunction, isolated tricuspid valve surgery may be considered(Level of Evidence: C-LD)
6. In patients with signs and symptoms of right-sided HF and severe TR (Stage D) who have undergone previous left-sided valve surgery, reoperation with isolated tricuspid valve surgery may be considered in the absence of severe pulmonary hypertension or severe RV systolic dysfunction(Level of Evidence: B-NR) |
References
- ↑ Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W (February 2022). "2021 ESC/EACTS Guidelines for the management of valvular heart disease". Eur Heart J. 43 (7): 561–632. doi:10.1093/eurheartj/ehab395. PMID 34453165 Check
|pmid=value (help). - ↑ Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA; et al. (2014). "2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines". Circulation. doi:10.1161/CIR.0000000000000029. PMID 24589852.
- ↑ Yunoki, Kei; Naruko, Takahiko; Itoh, Akira; Ohashi, Junko; Fujimoto, Kohei; Shirai, Naoya; Shimamura, Koichi; Komatsu, Ryushi; Sakanoue, Yuji; Haze, Kazuo (2006). "Percutaneous Transcatheter Balloon Valvuloplasty for Bioprosthetic Tricuspid Valve Stenosis". Circulation. 114 (18). doi:10.1161/CIRCULATIONAHA.106.618611. ISSN 0009-7322.
- ↑ Orbe, Luis Calvo; Sobrino, Nicolas; Arcas, Ramón; Peinado, Rafael; Frutos, Araceli; Blazquez, Jose Rico; Maté, Isabel; Sobrino, Jose Antonio (1993). "Initial outcome of percutaneous balloon valvuloplasty in rheumatic tricuspid valve stenosis". The American Journal of Cardiology. 71 (4): 353–354. doi:10.1016/0002-9149(93)90808-P. ISSN 0002-9149.
- ↑ Rana G, Malhotra R, Sharma A, Kakouros N (2017). "Percutaneous Valvuloplasty for Bioprosthetic Tricuspid Valve Stenosis". Tex Heart Inst J. 44 (1): 43–49. doi:10.14503/THIJ-15-5408. PMC 5317359. PMID 28265212.
- ↑ Sobrino N, Calvo Orbe L, Merino JL, Peinado R, Mate I, Rico J; et al. (1995). "Percutaneous balloon valvuloplasty for concurrent mitral, aortic and tricuspid rheumatic stenosis". Eur Heart J. 16 (5): 711–3. doi:10.1093/oxfordjournals.eurheartj.a060979. PMID 7588907.
- ↑ Ribeiro, Paulo A.; Zaibag, Muayed Al; Kasab, Saad Al; Idris, Mohamed; Halim, Murtada; Abdullah, Moheeb; Shahed, Maie (1988). "Percutaneous double balloon valvotomy for rheumatic tricuspid stenosis". The American Journal of Cardiology. 61 (8): 660–662. doi:10.1016/0002-9149(88)90790-4. ISSN 0002-9149.
- ↑ Egred, M.; Albouaini, K.; Morrison, W.L. (2006). "Balloon Valvuloplasty of a Stenosed Bioprosthetic Tricuspid Valve". Circulation. 113 (18). doi:10.1161/CIRCULATIONAHA.105.568238. ISSN 0009-7322.
- ↑ Patel TM, Dani SI, Shah SC, Patel TK (1996). "Tricuspid balloon valvuloplasty: a more simplified approach using inoue balloon". Cathet Cardiovasc Diagn. 37 (1): 86–8. doi:10.1002/(SICI)1097-0304(199601)37:1<86::AID-CCD23>3.0.CO;2-T. PMID 8770490.
- ↑ "Balloon Valvuloplasty for Bioprosthetic Tricuspid Valve Stenosis".
- ↑ "StatPearls". 2020. PMID 29763166.
- ↑ Kunadian B, Vijayalakshmi K, Balasubramanian S, Dunning J (2007). "Should the tricuspid valve be replaced with a mechanical or biological valve?". Interact Cardiovasc Thorac Surg. 6 (4): 551–7. doi:10.1510/icvts.2007.159277. PMID 17669933.
- ↑ Vassileva CM, Shabosky J, Boley T, Markwell S, Hazelrigg S (2012). "Tricuspid valve surgery: the past 10 years from the Nationwide Inpatient Sample (NIS) database". J Thorac Cardiovasc Surg. 143 (5): 1043–9. doi:10.1016/j.jtcvs.2011.07.004. PMID 21872283.
- ↑ Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F; et al. (2021). "2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines". Circulation. 143 (5): e72–e227. doi:10.1161/CIR.0000000000000923. PMID 33332150 Check
|pmid=value (help).