Triazolam: Difference between revisions
No edit summary |
No edit summary |
||
| Line 169: | Line 169: | ||
*Comparison of the cited figures, however, can provide the prescriber with some basis for estimating the relative contributions of drug and nondrug factors to the untoward event incidence rate in the population studied. Even this use must be approached cautiously, as a drug may relieve a symptom in one patient while inducing it in others. (For example, an [[anticholinergic]], [[anxiolytic]] drug may relieve dry mouth [a sign of [[anxiety]]] in some subjects but induce it [an untoward event] in others.) | *Comparison of the cited figures, however, can provide the prescriber with some basis for estimating the relative contributions of drug and nondrug factors to the untoward event incidence rate in the population studied. Even this use must be approached cautiously, as a drug may relieve a symptom in one patient while inducing it in others. (For example, an [[anticholinergic]], [[anxiolytic]] drug may relieve dry mouth [a sign of [[anxiety]]] in some subjects but induce it [an untoward event] in others.) | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*In addition to the relatively common (i.e., 1% or greater) untoward events enumerated above, the following adverse events have been reported less frequently (i.e., 0.9% to0.5%): [[euphoria]], [[tachycardia]], tiredness, confusional states/memory impairment, cramps/pain, [[depression]], visual disturbances. | *In addition to the relatively common (i.e., 1% or greater) untoward events enumerated above, the following adverse events have been reported less frequently (i.e., 0.9% to0.5%): [[euphoria]], [[tachycardia]], tiredness, confusional states/memory impairment, cramps/pain, [[depression]], visual disturbances. | ||
| Line 183: | Line 183: | ||
*Laboratory analyses were performed on all patients participating in the clinical program for triazolam. The following incidences of abnormalities were observed in patients receiving triazolam and the corresponding placebo group. None of these changes were considered to be of physiological significance. | *Laboratory analyses were performed on all patients participating in the clinical program for triazolam. The following incidences of abnormalities were observed in patients receiving triazolam and the corresponding placebo group. None of these changes were considered to be of physiological significance. | ||
: [[File:{{PAGENAME}}02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*When treatment with triazolam is protracted, periodic blood counts, [[urinalysis]], and blood chemistry analyses are advisable. | *When treatment with triazolam is protracted, periodic blood counts, [[urinalysis]], and blood chemistry analyses are advisable. | ||
| Line 393: | Line 393: | ||
*The structural formula is represented below: | *The structural formula is represented below: | ||
: [[File:{{PAGENAME}} | : [[File:{{PAGENAME}}07.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
*Each triazolam tablet, for oral administration, contains 0.125 mg or 0.25 mg of triazolam. Inactive ingredients: 0.125 mg—cellulose, corn starch, docusate sodium, lactose, magnesium stearate, silicon dioxide, sodium benzoate; 0.25 mg—cellulose, corn starch, docusate sodium, FD&C Blue No. 2, lactose, magnesium stearate, silicon dioxide, sodium benzoate. | *Each triazolam tablet, for oral administration, contains 0.125 mg or 0.25 mg of triazolam. Inactive ingredients: 0.125 mg—cellulose, corn starch, docusate sodium, lactose, magnesium stearate, silicon dioxide, sodium benzoate; 0.25 mg—cellulose, corn starch, docusate sodium, FD&C Blue No. 2, lactose, magnesium stearate, silicon dioxide, sodium benzoate. | ||
| Line 454: | Line 454: | ||
*"Sleep-driving" and other complex behaviors | *"Sleep-driving" and other complex behaviors | ||
:*There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when sedative-hypnotics are taken with alcohol or other central nervous system depressants. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative hypnotic. As with sleep-driving, patients usually do not remember these events. | :*There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when sedative-hypnotics are taken with alcohol or other central nervous system depressants. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative hypnotic. As with sleep-driving, patients usually do not remember these events. | ||
: [[File:{{PAGENAME}}03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
| Line 496: | Line 498: | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}04.png|This image is provided by the National Library of Medicine. | ||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}05.png|This image is provided by the National Library of Medicine. | |||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}06.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
Revision as of 15:55, 21 November 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Triazolam is a triazolobenzodiazepine hypnotic agent that is FDA approved for the {{{indicationType}}} of insomnia. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Insomnia
- Triazolam is indicated for the short-term treatment of insomnia (generally 7–10 days). Use for more than 2–3 weeks requires complete reevaluation of the patient.
- Prescriptions for triazolam should be written for short-term use (7–10 days) and it should not be prescribed in quantities exceeding a 1-month supply.
- It is important to individualize the dosage of triazolam tablets for maximum beneficial effect and to help avoid significant adverse effects.
- The recommended dose for most adults is 0.25 mg before retiring. A dose of 0.125 mg may be found to be sufficient for some patients (e.g., low body weight). A dose of 0.5 mg should be used only for exceptional patients who do not respond adequately to a trial of a lower dose since the risk of several adverse reactions increases with the size of the dose administered. A dose of 0.5 mg should not be exceeded.
- In geriatric and/or debilitated patients the recommended dosage range is 0.125 mg to 0.25 mg. Therapy should be initiated at 0.125 mg in these groups and the 0.25 mg dose should be used only for exceptional patients who do not respond to a trial of the lower dose. A dose of 0.25 mg should not be exceeded in these patients.
- As with all medications, the lowest effective dose should be used.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Triazolam in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Triazolam in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Triazolam in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Triazolam in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Triazolam in pediatric patients.
Contraindications
- Triazolam tablets are contraindicated in patients with known hypersensitivity to this drug or other benzodiazepines.
- Benzodiazepines may cause fetal damage when administered during pregnancy. An increased risk of congenital malformations associated with the use of diazepam and chlordiazepoxide during the first trimester of pregnancy has been suggested in several studies. Transplacental distribution has resulted in neonatal CNS depression following the ingestion of therapeutic doses of a benzodiazepine hypnotic during the last weeks of pregnancy.
- Triazolam is contraindicated in pregnant women. If there is a likelihood of the patient becoming pregnant while receiving triazolam, she should be warned of the potential risk to the fetus. Patients should be instructed to discontinue the drug prior to becoming pregnant. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered.
- Triazolam is contraindicated with medications that significantly impair the oxidative metabolism mediated by cytochrome P450 3A (CYP 3A) including ketoconazole, itraconazole, nefazodone, and several HIV protease inhibitors, (see WARNINGS and PRECAUTIONS–Drug Interactions).
Warnings
- Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative-hypnotic drugs. Because some of the important adverse effects of sedative-hypnotics appear to be dose related (see Precautions and Dosage and Administration), it is important to use the smallest possible effective dose, especially in the elderly.
- Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naïve as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with sedative-hypnotics alone at therapeutic doses, the use of alcohol and other CNS depressants with sedative-hypnotics appears to increase the risk of such behaviors, as does the use of sedative-hypnotics at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of sedative-hypnotics should be strongly considered for patients who report a "sleep-driving" episode.
- Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
- Severe anaphylactic and anaphylactoid reactions
- Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including triazolam. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with triazolam should not be rechallenged with the drug.
- Central nervous system manifestations
- An increase in daytime anxiety has been reported for triazolam after as few as 10 days of continuous use. In some patients this may be a manifestation of interdose withdrawal. If increased daytime anxiety is observed during treatment, discontinuation of treatment may be advisable.
- A variety of abnormal thinking and behavior changes have been reported to occur in association with the use of benzodiazepine hypnotics including triazolam. Some of these changes may be characterized by decreased inhibition, eg, aggressiveness and extroversion that seem excessive, similar to that seen with alcohol and other CNS depressants (eg, sedative/hypnotics). Other kinds of behavioral changes have also been reported, for example, bizarre behavior, agitation, hallucinations, depersonalization. In primarily depressed patients, the worsening of depression, including suicidal thinking, has been reported in association with the use of benzodiazepines.
- It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
- Because of its depressant CNS effects, patients receiving triazolam should be cautioned against engaging in hazardous occupations requiring complete mental alertness such as operating machinery or driving a motor vehicle. For the same reason, patients should be cautioned about the concomitant ingestion of alcohol and other CNS depressant drugs during treatment with triazolam tablets.
- As with some, but not all benzodiazepines, anterograde amnesia of varying severity and paradoxical reactions have been reported following therapeutic doses of triazolam. Data from several sources suggest that anterograde amnesia may occur at a higher rate with triazolam than with other benzodiazepine hypnotics.
- Triazolam interaction with drugs that inhibit metabolism via cytochrome P450 3A
- The initial step in triazolam metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP 3A). Drugs that inhibit this metabolic pathway may have a profound effect on the clearance of triazolam. Consequently, triazolam should be avoided in patients receiving very potent inhibitors of CYP 3A. With drugs inhibiting CYP 3A to a lesser but still significant degree, triazolam should be used only with caution and consideration of appropriate dosage reduction. For some drugs, an interaction with triazolam has been quantified with clinical data; for other drugs, interactions are predicted from in vitro data and/or experience with similar drugs in the same pharmacologic class.
- The following are examples of drugs known to inhibit the metabolism of triazolam and/or related benzodiazepines, presumably through inhibition of CYP 3A.
- Potent CYP 3A inhibitors
- Potent inhibitors of CYP 3A that should not be used concomitantly with triazolam include ketoconazole, itraconazole, nefazodone and several HIV protease inhibitors including ritonavir, indinavir, nelfinavir, saquinavir and lopinavir. Although data concerning the effects of azole-type antifungal agents other than ketoconazole and itraconazole on triazolam metabolism are not available, they should be considered potent CYP 3A inhibitors, and their coadministration with triazolam is not recommended.
- Drugs demonstrated to be CYP 3A inhibitors on the basis of clinical studies involving triazolam (caution and consideration of dose reduction are recommended during coadministration with triazolam)
- Macrolide Antibiotics
- Coadministration of erythromycin increased the maximum plasma concentration of triazolam by 46%, decreased clearance by 53%, and increased half-life by 35%; caution and consideration of appropriate triazolam dose reduction are recommended. Similar caution should be observed during coadministration with clarithromycin and other macrolide antibiotics.
- Cimetidine
- Coadministration of cimetidine increased the maximum plasma concentration of triazolam by 51%, decreased clearance by 55%, and increased half-life by 68%; caution and consideration of appropriate triazolam dose reduction are recommended.
- Other drugs possibly affecting triazolam metabolism
- Other drugs possibly affecting triazolam metabolism by inhibition of CYP 3A are discussed in the PRECAUTIONS section.
Precautions
- In elderly and/or debilitated patients it is recommended that treatment with triazolam tablets be initiated at 0.125 mg to decrease the possibility of development of oversedation, dizziness, or impaired coordination.
- Some side effects reported in association with the use of triazolam appear to be dose related. These include drowsiness, dizziness, light-headedness, and amnesia.
- The relationship between dose and what may be more serious behavioral phenomena is less certain. Specifically, some evidence, based on spontaneous marketing reports, suggests that confusion, bizarre or abnormal behavior, agitation, and hallucinations may also be dose related, but this evidence is inconclusive. In accordance with good medical practice it is recommended that therapy be initiated at the lowest effective dose.
- Cases of "traveler's amnesia" have been reported by individuals who have taken triazolam to induce sleep while traveling, such as during an airplane flight. In some of these cases, insufficient time was allowed for the sleep period prior to awakening and before beginning activity. Also, the concomitant use of alcohol may have been a factor in some cases.
- Caution should be exercised if triazolam is prescribed to patients with signs or symptoms of depression that could be intensified by hypnotic drugs. Suicidal tendencies may be present in such patients and protective measures may be required. Intentional over-dosage is more common in these patients, and the least amount of drug that is feasible should be available to the patient at any one time.
- The usual precautions should be observed in patients with impaired renal or hepatic function, chronic pulmonary insufficiency, and sleep apnea. In patients with compromised respiratory function, respiratory depression and apnea have been reported infrequently.
Adverse Reactions
Clinical Trials Experience
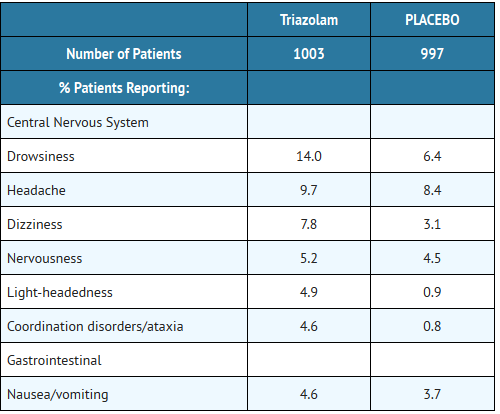
- During placebo-controlled clinical studies in which 1,003 patients received triazolam tablets, the most troublesome side effects were extensions of the pharmacologic activity of triazolam, eg, drowsiness, dizziness, or light-headedness.
- The figures cited below are estimates of untoward clinical event incidence among subjects who participated in the relatively short duration (i.e., 1 to 42 days) placebo-controlled clinical trials of triazolam. The figures cannot be used to predict precisely the incidence of untoward events in the course of usual medical practice where patient characteristics and other factors often differ from those in clinical trials. These figures cannot be compared with those obtained from other clinical studies involving related drug products and placebo, as each group of drug trials is conducted under a different set of conditions.
- Comparison of the cited figures, however, can provide the prescriber with some basis for estimating the relative contributions of drug and nondrug factors to the untoward event incidence rate in the population studied. Even this use must be approached cautiously, as a drug may relieve a symptom in one patient while inducing it in others. (For example, an anticholinergic, anxiolytic drug may relieve dry mouth [a sign of anxiety] in some subjects but induce it [an untoward event] in others.)
- In addition to the relatively common (i.e., 1% or greater) untoward events enumerated above, the following adverse events have been reported less frequently (i.e., 0.9% to0.5%): euphoria, tachycardia, tiredness, confusional states/memory impairment, cramps/pain, depression, visual disturbances.
- Rare (i.e., less than 0.5%) adverse reactions included constipation, taste alterations, diarrhea, dry mouth, dermatitis/allergy, dreaming/nightmares, insomnia, paresthesia, tinnitus, dysesthesia, weakness, congestion, death from hepatic failure in a patient also receiving diuretic drugs.
- In addition to these untoward events for which estimates of incidence are available, the following adverse events have been reported in association with the use of triazolam and other benzodiazepines: amnestic symptoms (anterograde amnesia with appropriate or inappropriate behavior), confusional states (disorientation, derealization, depersonalization, and/or clouding of consciousness), dystonia, anorexia, fatigue, sedation, slurred speech, jaundice, pruritus, dysarthria, changes in libido, menstrual irregularities, incontinence, and urinary retention. Other factors may contribute to some of these reactions, eg, concomitant intake of alcohol or other drugs, sleep deprivation, an abnormal premorbid state, etc.
- Other events reported include: paradoxical reactions such as stimulation, mania, an agitational state (restlessness, irritability, and excitation), increased muscle spasticity, sleep disturbances, hallucinations, delusions, aggressiveness, falling, somnambulism, syncope, inappropriate behavior and other adverse behavioral effects. Should these occur, use of the drug should be discontinued.
- The following events have also been reported: chest pain, burning tongue/glossitis/stomatitis.
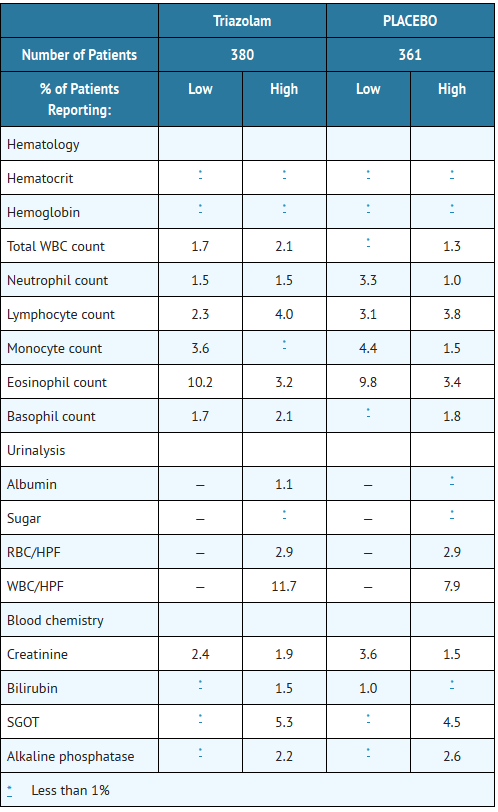
- Laboratory analyses were performed on all patients participating in the clinical program for triazolam. The following incidences of abnormalities were observed in patients receiving triazolam and the corresponding placebo group. None of these changes were considered to be of physiological significance.
- When treatment with triazolam is protracted, periodic blood counts, urinalysis, and blood chemistry analyses are advisable.
- Minor changes in EEG patterns, usually low-voltage fast activity, have been observed in patients during therapy with triazolam and are of no known significance.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Triazolam in the drug label.
Drug Interactions
- Both pharmacodynamic and pharmacokinetic interactions have been reported with benzodiazepines. In particular, triazolam produces additive CNS depressant effects when coadministered with other psychotropic medications, anticonvulsants, antihistamines, ethanol, and other drugs which themselves produce CNS depression.
- Drugs that inhibit triazolam metabolism via cytochrome P450 3A
- The initial step in triazolam metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP 3A). Drugs which inhibit this metabolic pathway may have a profound effect on the clearance of triazolam. Triazolam is contraindicated with ketoconzaole, itraconazole, nefazodone, and several HIV protease inhibitors.
- Drugs and other substances demonstrated to be CYP 3A inhibitors of possible clinical significance on the basis of clinical studies involving triazolam.
- Isoniazid
- Coadministration of isoniazid increased the maximum plasma concentration of triazolam by 20%, decreased clearance by 42%, and increased half-life by 31%.
- Oral contraceptives
- Coadministration of oral contraceptives increased maximum plasma concentration by 6%, decreased clearance by 32%, and increased half-life by 16%.
- Grapefruit juice
- Coadministration of grapefruit juice increased the maximum plasma concentration of triazolam by 25%, increased the area under the concentration curve by 48%, and increased half-life by 18%.
- Drugs demonstrated to be CYP 3A inhibitors on the basis of clinical studies involving benzodiazepines metabolized similarly to triazolam or on the basis of in vitro studies with triazolam or other benzodiazepines (caution is recommended during coadministration with triazolam)
- Available data from clinical studies of benzodiazepines other than triazolam suggest a possible drug interaction with triazolam for the following: fluvoxamine, diltiazem, and verapamil. Data from in vitro studies of triazolam suggest a possible drug interaction with triazolam for the following: sertraline and paroxetine. Data from in vitro studies of benzodiazepines other than triazolam suggest a possible drug interaction with triazolam for the following: ergotamine, cyclosporine, amiodarone, nicardipine, and nifedipine. Caution is recommended during coadministration of any of these drugs with triazolam.
- Drugs that affect triazolam pharmacokinetics by other mechanisms
- Ranitidine
- Coadministration of ranitidine increased the maximum plasma concentration of triazolam by 30%, increased the area under the concentration curve by 27%, and increased half-life by 3.3%. Caution is recommended during coadministration with triazolam.
Use in Specific Populations
Pregnancy
- Pregnancy Category X
- Teratogenic effects
- Pregnancy category X (see CONTRAINDICATIONS).
- Non-teratogenic effects
- It is to be considered that the child born of a mother who is on benzodiazepines may be at some risk for withdrawal symptoms from the drug, during the postnatal period. Also, neonatal flaccidity has been reported in an infant born of a mother who had been receiving benzodiazepines.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Triazolam in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Triazolam during labor and delivery.
Nursing Mothers
- Human studies have not been performed; however, studies in rats have indicated that triazolam and its metabolites are secreted in milk. Therefore, administration of triazolam to nursing mothers is not recommended.
Pediatric Use
- Safety and effectiveness of triazolam in individuals below 18 years of age have not been established.
Geriatic Use
- The elderly are especially susceptible to the dose related adverse effects of triazolam. They exhibit higher plasma triazolam concentrations due to reduced clearance of the drug as compared with younger subjects at the same dose. To minimize the possibility of development of oversedation, the smallest effective dose should be used.
Gender
There is no FDA guidance on the use of Triazolam with respect to specific gender populations.
Race
There is no FDA guidance on the use of Triazolam with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Triazolam in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Triazolam in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Triazolam in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Triazolam in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Triazolam in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Triazolam in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Because of the potency of triazolam, some manifestations of overdosage may occur at 2 mg, four times the maximum recommended therapeutic dose (0.5 mg).
- Manifestations of overdosage with triazolam tablets include somnolence, confusion, impaired coordination, slurred speech, and ultimately, coma. Respiratory depression and apnea have been reported with overdosages of triazolam. Seizures have occasionally been reported after overdosages.
- Death has been reported in association with overdoses of triazolam by itself, as it has with other benzodiazepines. In addition, fatalities have been reported in patients who have overdosed with a combination of a single benzodiazepine, including triazolam, and alcohol; benzodiazepine and alcohol levels seen in some of these cases have been lower than those usually associated with reports of fatality with either substance alone.
Management
- As in all cases of drug overdosage, respiration, pulse, and blood pressure should be monitored and supported by general measures when necessary. Immediate gastric lavage should be performed. An adequate airway should be maintained. Intravenous fluids may be administered.
- Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose.
- Experiments in animals have indicated that cardiopulmonary collapse can occur with massive intravenous doses of triazolam. This could be reversed with positive mechanical respiration and the intravenous infusion of norepinephrine bitartrate or metaraminol bitartrate. Hemodialysis and forced diuresis are probably of little value. As with the management of intentional overdosage with any drug, the physician should bear in mind that multiple agents may have been ingested by the patient.
- The oral LD50 in mice is greater than 1,000 mg/kg and in rats is greater than 5,000 mg/kg.
Chronic Overdose
There is limited information regarding Chronic Overdose of Triazolam in the drug label.
Pharmacology
| |
| |
Triazolam
| |
| Systematic (IUPAC) name | |
| 8-chloro-6-(2-chlorophenyl)-1-methyl-4H- [1,2,4]triazolo[4,3-a][1,4]benzodiazepine | |
| Identifiers | |
| CAS number | |
| ATC code | N05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 343.2 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 44% (oral), 53% (sublingual) |
| Metabolism | Hepatic |
| Half life | 1.5–5.5 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
X (US) |
| Legal status |
Schedule IV(US) |
| Routes | Oral |
Mechanism of Action
- Triazolam is a triazolobenzodiazepine hypnotic drug that increases the duration of sleep and decreases the number of nocturnal awakenings and sleep latency.
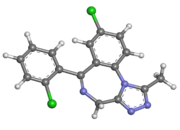
Structure
- Triazolam is a triazolobenzodiazepine hypnotic agent.
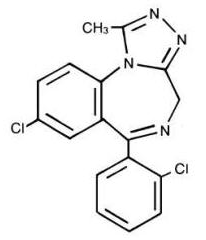
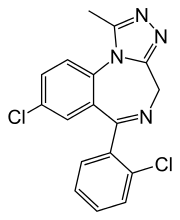
- Triazolam is a white crystalline powder, soluble in alcohol and poorly soluble in water. It has a molecular weight of 343.21.
- The chemical name for triazolam is 8-chloro-6-(o-chlorophenyl)-1-methyl-4H-s-triazolo-[4,3-α] [1,4] benzodiazepine.
- The structural formula is represented below:
- Each triazolam tablet, for oral administration, contains 0.125 mg or 0.25 mg of triazolam. Inactive ingredients: 0.125 mg—cellulose, corn starch, docusate sodium, lactose, magnesium stearate, silicon dioxide, sodium benzoate; 0.25 mg—cellulose, corn starch, docusate sodium, FD&C Blue No. 2, lactose, magnesium stearate, silicon dioxide, sodium benzoate.
Pharmacodynamics
- In sleep laboratory studies, triazolam tablets significantly decreased sleep latency, increased the duration of sleep, and decreased the number of nocturnal awakenings. After 2 weeks of consecutive nightly administration, the drug's effect on total wake time is decreased, and the values recorded in the last third of the night approach baseline levels. On the first and/or second night after drug discontinuance (first or second post-drug night), total time asleep, percentage of time spent sleeping, and rapidity of falling asleep frequently were significantly less than on baseline (predrug) nights. This effect is often called "rebound" insomnia.
- The type and duration of hypnotic effects and the profile of unwanted effects during administration of benzodiazepine drugs may be influenced by the biologic half-life of administered drug and any active metabolites formed. When half-lives are long, the drug or metabolites may accumulate during periods of nightly administration and be associated with impairments of cognitive and motor performance during waking hours; the possibility of interaction with other psychoactive drugs or alcohol will be enhanced. In contrast, if half-lives are short, the drug and metabolites will be cleared before the next dose is ingested, and carry-over effects related to excessive sedation or CNS depression should be minimal or absent. However, during nightly use for an extended period pharmacodynamic tolerance or adaptation to some effects of benzodiazepine hypnotics may develop. If the drug has a short half-life of elimination, it is possible that a relative deficiency of the drug or its active metabolites (ie, in relationship to the receptor site) may occur at some point in the interval between each night's use. This sequence of events may account for two clinical findings reported to occur after several weeks of nightly use of rapidly eliminated benzodiazepine hypnotics: 1) increased wakefulness during the last third of the night and 2) the appearance of increased daytime anxiety after 10 days of continuous treatment.
- In a study of elderly (62–83 years old) versus younger subjects (21–41 years old) who received triazolam at the same dose levels (0.125 mg and 0.25 mg), the elderly experienced both greater sedation and impairment of psychomotor performance. These effects resulted largely from higher plasma concentrations of triazolam in the elderly.
Pharmacokinetics
- Triazolam is a hypnotic with a short mean plasma half-life reported to be in the range of 1.5 to 5.5 hours. In normal subjects treated for 7 days with four times the recommended dosage, there was no evidence of altered systemic bioavailability, rate of elimination, or accumulation. Peak plasma levels are reached within 2 hours following oral administration. Following recommended doses of triazolam tablets, triazolam peak plasma levels in the range of 1 to 6 ng/mL are seen. The plasma levels achieved are proportional to the dose given.
- Triazolam and its metabolites, principally as conjugated glucuronides, which are presumably inactive, are excreted primarily in the urine. Only small amounts of unmetabolized triazolam appear in the urine. The two primary metabolites accounted for 79.9% of urinary excretion. Urinary excretion appeared to be biphasic in its time course.
- Triazolam tablets 0.5 mg, in two separate studies, did not affect the prothrombin times or plasma warfarin levels in male volunteers administered sodium warfarin orally.
- Extremely high concentrations of triazolam do not displace bilirubin bound to human serum albumin in vitro.
- Triazolam 14C was administered orally to pregnant mice. Drug-related material appeared uniformly distributed in the fetus with 14C concentrations approximately the same as in the brain of the mother.
Nonclinical Toxicology
- No evidence of carcinogenic potential was observed in mice during a 24-month study with triazolam in doses up to 4,000 times the human dose.
Clinical Studies
There is limited information regarding Clinical Studies of Triazolam in the drug label.
How Supplied
- Triazolam tablets are available in the following strengths and package sizes:
- 0.125 mg (white,, imprinted G3717):
- 10–10 Tablet Bottles NDC 59762-3717-4
- 0.125 mg (white,, imprinted G3717):
- 0.25 mg (powder blue, scored, imprinted G3718):
- 10–10 Tablet Bottles NDC 59762-3718-4
- Bottles of 500 NDC 59762-3718-3
- 0.25 mg (powder blue, scored, imprinted G3718):
- Store at controlled room temperature 20° to 25°C (68° to 77°F).
Storage
There is limited information regarding Triazolam Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Triazolam |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Triazolam |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- "Sleep-driving" and other complex behaviors
- There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when sedative-hypnotics are taken with alcohol or other central nervous system depressants. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative hypnotic. As with sleep-driving, patients usually do not remember these events.
Precautions with Alcohol
- Alcohol-Triazolam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- TRIAZOLAM®[1]
Look-Alike Drug Names
There is limited information regarding Triazolam Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Triazolam |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Triazolam |Label Name=Triazolam04.png
}}
{{#subobject:
|Label Page=Triazolam |Label Name=Triazolam05.png
}}
{{#subobject:
|Label Page=Triazolam |Label Name=Triazolam06.png
}}