Tongue cancer surgery: Difference between revisions
Simrat Sarai (talk | contribs) |
No edit summary |
||
| (13 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Tongue cancer}} | {{Tongue cancer}} | ||
{{CMG}}{{AE}}{{Simrat}} | {{CMG}}; {{AE}} {{Simrat}} {{MAD}} | ||
==Overview== | ==Overview== | ||
Surgery is the mainstay of treatment for tongue cancer. | Surgery is the mainstay of treatment for tongue cancer. Radical approach is required in larger lesions, impaired [[tongue]] mobility, deep tongue infiltration, floor-of-mouth extension. A '''partial glossectomy''' with negative margins can preserve speech and swallowing for most stage I and II lesions of the oral tongue. Partial glossectomy is commonly required for advanced disease.Total glossectomy is required in cases where bilateral lingual arteries are involved by cancer. In these cases, postoperative [[radiotherapy]] or chemoradiotherapy, appears to improve disease control compared with surgery alone. Elective treatment of the neck in patients with stage I and II [[oral cavity]] cancer is not well established. Studies recommend a '''[[tumor]] thickness cutoff of 4 mm''' as a threshold for elective [[neck dissection]]. | ||
==Surgery== | ==Surgery== | ||
* | *The ideal surgical approach to oral tongue [[Tumor|tumors]] depends on the [[tumor]] size and the involvement of adjacent structures. | ||
*For most small T1 and T2 lesions confined to the [[tongue]], excision is possible with a hemiglossectory or partial hemiglossectomy. | |||
*Reconstruction of the [[tongue]] depends on the size of the defect. When less than a third of the tongue has been resected primary closure is possible.<ref name="pmid8567334">{{cite journal| author=Fujita M, Hirokawa Y, Kashiwado K, Akagi Y, Kashimoto K, Kiriu H et al.| title=An analysis of mandibular bone complications in radiotherapy for T1 and T2 carcinoma of the oral tongue. | journal=Int J Radiat Oncol Biol Phys | year= 1996 | volume= 34 | issue= 2 | pages= 333-9 | pmid=8567334 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8567334 }} </ref> | |||
* | *Radical approach is required in: | ||
* | *Larger lesions | ||
*Impaired [[tongue]] mobility | |||
*Deep tongue infiltration | |||
*Floor-of-mouth extension | |||
==== Approach ==== | |||
*The tongue may be approached through a lateral pharyngotomy. | |||
*A '''partial glossectomy''' with negative margins can preserve speech and swallowing for most stage I and II lesions of the oral tongue. | |||
*Partial glossectomy is commonly required for advanced disease. | |||
*Total glossectomy is required in cases where bilateral lingual arteries are involved by cancer. In these cases, postoperative [[radiotherapy]] or chemoradiotherapy, appears to improve disease control compared with surgery alone.<ref name="pmid8056581">{{cite journal| author=Fein DA, Mendenhall WM, Parsons JT, McCarty PJ, Stringer SP, Million RR et al.| title=Carcinoma of the oral tongue: a comparison of results and complications of treatment with radiotherapy and/or surgery. | journal=Head Neck | year= 1994 | volume= 16 | issue= 4 | pages= 358-65 | pmid=8056581 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8056581 }} </ref> | |||
*A mandibulotomy may be required for access if the [[mandible]] is free of [[Tumor|tumor.]] | |||
*Consider resection of the [[mandible]] in case of tumor involves or extends to the [[gingiva]]. | |||
*[[Free flap|Free-flap]] reconstruction is required for larger defects. | |||
*Larger lesions which cross the midline are usually not resected due to the operation being poorly tolerated.<ref name="pmid9486601">{{cite journal| author=Matsuura K, Hirokawa Y, Fujita M, Akagi Y, Ito K| title=Treatment results of stage I and II oral tongue cancer with interstitial brachytherapy: maximum tumor thickness is prognostic of nodal metastasis. | journal=Int J Radiat Oncol Biol Phys | year= 1998 | volume= 40 | issue= 3 | pages= 535-9 | pmid=9486601 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9486601 }} </ref> | |||
*Total [[laryngectomy]] may also be required to prevent [[aspiration]]. | |||
*Assessing surgical resection margins can be difficult. Therefore, close deep surgical margins should be interpreted with caution and more aggressive treatment may be indicated compared with close radial [[mucosal]] margins or close margins in other disease sites. | |||
==== Management of the neck ==== | |||
* Elective treatment of the neck in patients with stage I and II oral cavity cancer is not well established.<ref name="pmid21901703">{{cite journal| author=Bessell A, Glenny AM, Furness S, Clarkson JE, Oliver R, Conway DI et al.| title=Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. | journal=Cochrane Database Syst Rev | year= 2011 | volume= | issue= 9 | pages= CD006205 | pmid=21901703 | doi=10.1002/14651858.CD006205.pub3 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21901703 }}</ref> | |||
* Most reports have found that increasing '''[[tumor]] thickness''' is associated with an increased risk of [[occult]] [[Metastasis|metastases]] and reduced survival.<ref name="pmid19197973">{{cite journal| author=Huang SH, Hwang D, Lockwood G, Goldstein DP, O'Sullivan B| title=Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies. | journal=Cancer | year= 2009 | volume= 115 | issue= 7 | pages= 1489-97 | pmid=19197973 | doi=10.1002/cncr.24161 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19197973 }}</ref> | |||
* Studies recommend a '''[[tumor]] thickness cutoff of 4 mm''' as a threshold for elective neck dissection.<ref name="pmid26027881">{{cite journal| author=D'Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R et al.| title=Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. | journal=N Engl J Med | year= 2015 | volume= 373 | issue= 6 | pages= 521-9 | pmid=26027881 | doi=10.1056/NEJMoa1506007 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26027881 }}</ref> | |||
* The benefit was present in all subgroups, except for those with a primary [[tumor]] depth ≤3 mm. | |||
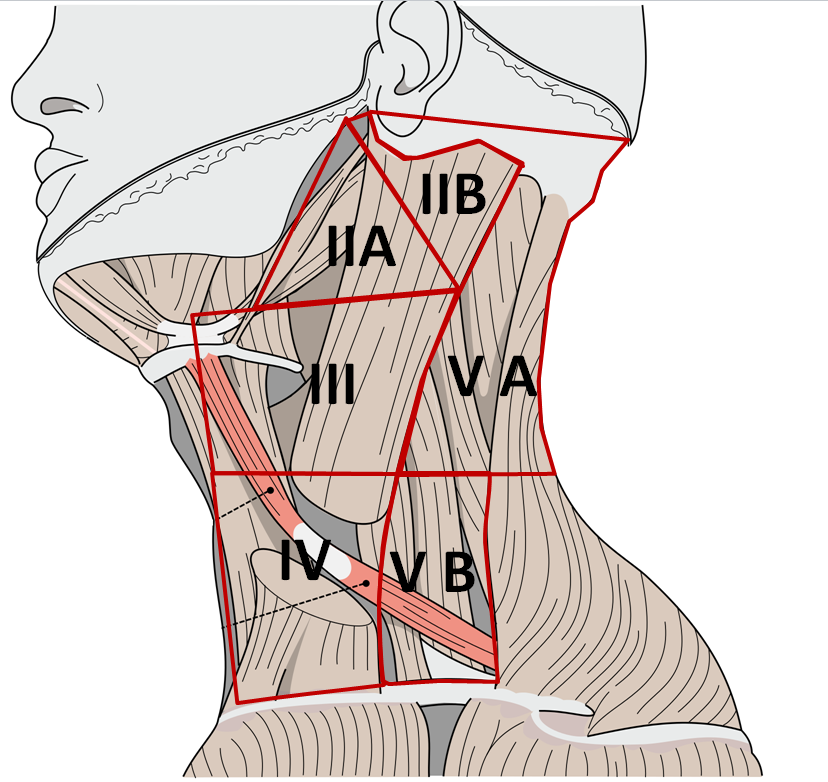
* An ipsilateral selective neck dissection, levels I to III/IV, for stage I cancers with greater than 3 mm of invasion and for most stage II disease, except minimally invasive primary tumors. | |||
* Levels IIB and IV are dissected at the discretion of the surgeon. | |||
* Patients with primary tumors close to or involving the midline should be managed with bilateral [[neck dissection]].<ref name="pmid26597442">{{cite journal| author=Schilling C, Stoeckli SJ, Haerle SK, Broglie MA, Huber GF, Sorensen JA et al.| title=Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. | journal=Eur J Cancer | year= 2015 | volume= 51 | issue= 18 | pages= 2777-84 | pmid=26597442 | doi=10.1016/j.ejca.2015.08.023 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26597442 }}</ref> | |||
* [[Sentinel lymph node]] biopsy may be an important option between observation and [[neck dissection]] in patients with intermediate-thickness [[Tumor|tumors]].<ref name="pmid26040238">{{cite journal| author=Pedersen NJ, Jensen DH, Hedbäck N, Frendø M, Kiss K, Lelkaitis G et al.| title=Staging of early lymph node metastases with the sentinel lymph node technique and predictive factors in T1/T2 oral cavity cancer: A retrospective single-center study. | journal=Head Neck | year= 2016 | volume= 38 Suppl 1 | issue= | pages= E1033-40 | pmid=26040238 | doi=10.1002/hed.24153 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26040238 }}</ref> | |||
* The technique may be most applicable to patients with primary [[Tumor|tumors]] less than 3 mm in depth that have an intermediate risk of [[Lymph node|lymph node metastasis]], and larger primary [[Tumor|tumors]]. | |||
* A negative [[Sentinel lymph node|sentinel lymph node biopsy]] may replace planned [[Neck dissection|neck dissection.]]<ref name="pmid27878928">{{cite journal| author=Abdul-Razak M, Chung H, Wong E, Palme C, Veness M, Farlow D et al.| title=Sentinel lymph node biopsy for early oral cancers: Westmead Hospital experience. | journal=ANZ J Surg | year= 2017 | volume= 87 | issue= 1-2 | pages= 65-69 | pmid=27878928 | doi=10.1111/ans.13853 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27878928 }}</ref> | |||
* [[Brachytherapy]] can be used in large lesions. The patient is then monitored postoperatively for 48 hours, during which the oncologist proceeds with the [[brachytherapy]] dosimetry and implantation of [[Radioactive|radioactive seeds]] for periods of up to 72 hours. | |||
*The needles are then removed and the patient's recovery proceeds in-hospital. | |||
[[File:MED.png|300px|center|thumb|Neck dissection levels, source: modified from Image:Gray385.png modified by Uwe Gille - Image:Gray385.png, Public Domain, https://commons.wikimedia.org/w/index.php?curid=2492547]] | |||
== Video shows glossectomy steps == | |||
{{#ev:youtube|t6MFGg_vOkU}} | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
{{ | {{WH}} | ||
{{ | {{Ws}} | ||
Latest revision as of 18:01, 4 December 2017
|
Tongue cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tongue cancer surgery On the Web |
|
American Roentgen Ray Society Images of Tongue cancer surgery |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Simrat Sarai, M.D. [2] Mohammed Abdelwahed M.D[3]
Overview
Surgery is the mainstay of treatment for tongue cancer. Radical approach is required in larger lesions, impaired tongue mobility, deep tongue infiltration, floor-of-mouth extension. A partial glossectomy with negative margins can preserve speech and swallowing for most stage I and II lesions of the oral tongue. Partial glossectomy is commonly required for advanced disease.Total glossectomy is required in cases where bilateral lingual arteries are involved by cancer. In these cases, postoperative radiotherapy or chemoradiotherapy, appears to improve disease control compared with surgery alone. Elective treatment of the neck in patients with stage I and II oral cavity cancer is not well established. Studies recommend a tumor thickness cutoff of 4 mm as a threshold for elective neck dissection.
Surgery
- The ideal surgical approach to oral tongue tumors depends on the tumor size and the involvement of adjacent structures.
- For most small T1 and T2 lesions confined to the tongue, excision is possible with a hemiglossectory or partial hemiglossectomy.
- Reconstruction of the tongue depends on the size of the defect. When less than a third of the tongue has been resected primary closure is possible.[1]
- Radical approach is required in:
- Larger lesions
- Impaired tongue mobility
- Deep tongue infiltration
- Floor-of-mouth extension
Approach
- The tongue may be approached through a lateral pharyngotomy.
- A partial glossectomy with negative margins can preserve speech and swallowing for most stage I and II lesions of the oral tongue.
- Partial glossectomy is commonly required for advanced disease.
- Total glossectomy is required in cases where bilateral lingual arteries are involved by cancer. In these cases, postoperative radiotherapy or chemoradiotherapy, appears to improve disease control compared with surgery alone.[2]
- A mandibulotomy may be required for access if the mandible is free of tumor.
- Consider resection of the mandible in case of tumor involves or extends to the gingiva.
- Free-flap reconstruction is required for larger defects.
- Larger lesions which cross the midline are usually not resected due to the operation being poorly tolerated.[3]
- Total laryngectomy may also be required to prevent aspiration.
- Assessing surgical resection margins can be difficult. Therefore, close deep surgical margins should be interpreted with caution and more aggressive treatment may be indicated compared with close radial mucosal margins or close margins in other disease sites.
Management of the neck
- Elective treatment of the neck in patients with stage I and II oral cavity cancer is not well established.[4]
- Most reports have found that increasing tumor thickness is associated with an increased risk of occult metastases and reduced survival.[5]
- Studies recommend a tumor thickness cutoff of 4 mm as a threshold for elective neck dissection.[6]
- The benefit was present in all subgroups, except for those with a primary tumor depth ≤3 mm.
- An ipsilateral selective neck dissection, levels I to III/IV, for stage I cancers with greater than 3 mm of invasion and for most stage II disease, except minimally invasive primary tumors.
- Levels IIB and IV are dissected at the discretion of the surgeon.
- Patients with primary tumors close to or involving the midline should be managed with bilateral neck dissection.[7]
- Sentinel lymph node biopsy may be an important option between observation and neck dissection in patients with intermediate-thickness tumors.[8]
- The technique may be most applicable to patients with primary tumors less than 3 mm in depth that have an intermediate risk of lymph node metastasis, and larger primary tumors.
- A negative sentinel lymph node biopsy may replace planned neck dissection.[9]
- Brachytherapy can be used in large lesions. The patient is then monitored postoperatively for 48 hours, during which the oncologist proceeds with the brachytherapy dosimetry and implantation of radioactive seeds for periods of up to 72 hours.
- The needles are then removed and the patient's recovery proceeds in-hospital.
Video shows glossectomy steps
{{#ev:youtube|t6MFGg_vOkU}}
References
- ↑ Fujita M, Hirokawa Y, Kashiwado K, Akagi Y, Kashimoto K, Kiriu H; et al. (1996). "An analysis of mandibular bone complications in radiotherapy for T1 and T2 carcinoma of the oral tongue". Int J Radiat Oncol Biol Phys. 34 (2): 333–9. PMID 8567334.
- ↑ Fein DA, Mendenhall WM, Parsons JT, McCarty PJ, Stringer SP, Million RR; et al. (1994). "Carcinoma of the oral tongue: a comparison of results and complications of treatment with radiotherapy and/or surgery". Head Neck. 16 (4): 358–65. PMID 8056581.
- ↑ Matsuura K, Hirokawa Y, Fujita M, Akagi Y, Ito K (1998). "Treatment results of stage I and II oral tongue cancer with interstitial brachytherapy: maximum tumor thickness is prognostic of nodal metastasis". Int J Radiat Oncol Biol Phys. 40 (3): 535–9. PMID 9486601.
- ↑ Bessell A, Glenny AM, Furness S, Clarkson JE, Oliver R, Conway DI; et al. (2011). "Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment". Cochrane Database Syst Rev (9): CD006205. doi:10.1002/14651858.CD006205.pub3. PMID 21901703.
- ↑ Huang SH, Hwang D, Lockwood G, Goldstein DP, O'Sullivan B (2009). "Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity: a meta-analysis of reported studies". Cancer. 115 (7): 1489–97. doi:10.1002/cncr.24161. PMID 19197973.
- ↑ D'Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R; et al. (2015). "Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer". N Engl J Med. 373 (6): 521–9. doi:10.1056/NEJMoa1506007. PMID 26027881.
- ↑ Schilling C, Stoeckli SJ, Haerle SK, Broglie MA, Huber GF, Sorensen JA; et al. (2015). "Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer". Eur J Cancer. 51 (18): 2777–84. doi:10.1016/j.ejca.2015.08.023. PMID 26597442.
- ↑ Pedersen NJ, Jensen DH, Hedbäck N, Frendø M, Kiss K, Lelkaitis G; et al. (2016). "Staging of early lymph node metastases with the sentinel lymph node technique and predictive factors in T1/T2 oral cavity cancer: A retrospective single-center study". Head Neck. 38 Suppl 1: E1033–40. doi:10.1002/hed.24153. PMID 26040238.
- ↑ Abdul-Razak M, Chung H, Wong E, Palme C, Veness M, Farlow D; et al. (2017). "Sentinel lymph node biopsy for early oral cancers: Westmead Hospital experience". ANZ J Surg. 87 (1–2): 65–69. doi:10.1111/ans.13853. PMID 27878928.