Superior vena cava syndrome
Template:DiseaseDisorder infobox
| Cardiology Network |
 Discuss Superior vena cava syndrome further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Synonyms and Related Keywords: SVC syndrome
Overview
Superior vena cava syndrome (SVCS) is an array of symptoms caused by the impairment of blood flow through the superior vena cava (SVC) to the right atrium. Symptoms that prompt suspicion of this syndrome include dyspnea, coughing, and swelling of the face, neck, upper trunk, and extremities. In rare instances, patients may complain of hoarseness, chest pain, dysphagia, and hemoptysis. Physical signs that may be noted on presentation are neck vein distention, thoracic vein distention, edema of the face or upper extremities, plethora, and tachypnea. Rarely, cyanosis, Horner syndrome, and a paralyzed vocal cord may also be present.
SVCS is usually a sign of locally advanced bronchogenic carcinoma. Survival depends on the status of the patient’s disease. When small cell bronchogenic carcinoma is treated with chemotherapy, the median survival times with or without SVCS are almost identical (42 weeks or 40 weeks, respectively). The 24-month survival rate is 9% in patients without SVCS and 3% in those with the syndrome. When the malignancy is treated with radiation therapy, 46% of patients who have non-small cell lung cancer experience relief of symptoms compared with 62% of patients who have small cell bronchogenic carcinoma. The 2-year survival rate of 5% is almost the same for both groups.
Most non-Hodgkin lymphoma patients with SVCS respond to appropriate chemotherapy or to combined modality regimens.
Etymology
The Scottish obstetrician and anatomist, William Hunter, first described the entity in 1757, noting it as a complication of a syphilitic aortic aneurysm.
Epidemiology and Demographics
Most SVC syndromes in the present day are related to malignancy. An underlying malignancy is found in approximately 90% of patients.
Pathophysiology & Etiology
Since SVCS was first described by William Hunter in 1757, the spectrum of underlying conditions associated with it has shifted from tuberculosis and syphilitic aneurysms of the ascending aorta to malignant disorders. Almost 95% of SVCS cases described in published modern series are due to cancer; the most common cause is small cell bronchogenic carcinoma, followed by squamous cell carcinoma of the lung, adenocarcinoma of the lung, non-Hodgkin lymphoma, and large cell carcinoma of the lung. A nonmalignant cause of SVCS in cancer patients is thrombosis that is associated with intracaval catheters or pacemaker wires. A rare cause of SVCS is fibrosing mediastinitis, either idiopathic or associated with histoplasmosis. Additional rare causes of SVCS include metastatic germ cell neoplasms, metastatic breast cancer, colon cancer, Kaposi sarcoma, esophageal carcinoma, fibrous mesothelioma, Behçet syndrome, thymoma, substernal thyroid goiter, Hodgkin lymphoma, and sarcoidosis.
Knowledge of the anatomy of the SVC and its relationship to the surrounding lymph nodes is essential to understanding the development of the syndrome. The SVC is formed by the junction of the left and right brachiocephalic veins in the mid third of the mediastinum. The SVC extends caudally for 6 to 8 cm, coursing anterior to the right mainstem bronchus and terminating in the superior right atrium, and extends anteriorly to the right mainstem bronchus. The SVC is joined posteriorly by the azygos vein as it loops over the right mainstem bronchus and lies posterior to and to the right of the ascending aorta. The mediastinal parietal pleura is lateral to the SVC, creating a confined space, and the SVC is adjacent to the right paratracheal, azygous, right hilar, and subcarinal lymph node groups. The vessel itself is thin-walled, and the blood flowing therein is under low pressure. Thus, when the nodes or ascending aorta enlarge, the SVC is compressed, blood flow slows, and complete occlusion may occur.
The severity of the syndrome depends on the rapidity of onset of the obstruction and its location. The more rapid the onset, the more severe the symptoms because the collateral veins do not have time to distend to accommodate an increased blood flow. If the obstruction is above the entry of the azygos vein, the syndrome is less pronounced because the azygous venous system can readily distend to accommodate the shunted blood with less venous pressure developing in the head, arms, and upper thorax. If the obstruction is below the entry of the azygos vein, more florid symptoms and signs are seen because the blood must be returned to the heart via the upper abdominal veins and the inferior vena cava, which requires higher venous pressure.
One study suggested that the general recruitment of venous collaterals over time may lead to remission of the syndrome, although the SVC remains obstructed
Natural History
In the past, SVC syndrome was a medical emergency and empiric radiation was given to shrink the tumor. With the advent of better medical therapy for some lung cancers and lymphoma and the low morbidity associated with diagnostic procedures, this approach has fallen out of favor.
Signs and symptoms
The most common symptoms are these:
- Dyspnea
- Cough
- Swollen face, neck, upper body, and arms.
Less common symptoms include the following:
- Hoarse voice.
- Chest pain.
- Problems swallowing and/or talking.
- Coughing up blood.
- Swollen veins in the chest or neck.
- Bluish color to the skin.
- Drooping eyelid.
- Headache
- Facial plethora
- Lightheadedness
Superior vena cava syndrome usually presents more gradually with an increase in symptoms over time as malignancies increase in size or invasiveness.[1]
Diagnosis
Once SVCS is recognized, prompt clinical attention is important. A diagnosis should be established prior to initiating therapy for the following reasons:
- 75% of patients have symptoms and signs for longer than 1 week before seeking medical attention.
- Cancer patients diagnosed with SVCS do not die of the syndrome itself but rather from the extent of their underlying disease.
- 3% to 5% of the patients diagnosed with SVCS do not have cancer.
In the absence of tracheal obstruction, SVCS is unlikely to be a life-threatening oncologic emergency, and treatment prior to definitive diagnosis is not justified.
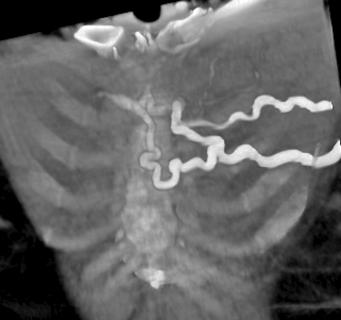
The initial evaluation of the patient should include a chest radiograph to look for mediastinal masses and associated findings, such as pleural effusion, lobar collapse, or cardiomegaly. Computed tomography (CT) scanning of the thorax yields the most useful diagnostic information and can define the anatomy of the involved mediastinal nodes. Venous patency and the presence of thrombi are assessed by using contrast and rapid scanning techniques. Depending on local expertise, contrast or nuclear venography, magnetic resonance imaging, and ultrasound may be valuable in assessing the site and nature of the obstruction.
If bronchogenic carcinoma is suspected, a sputum specimen should be obtained. If the sputum specimen is negative, a biopsy specimen should be taken from the most accessible site that is clinically involved with disease. The biopsy approach depends on the working diagnosis, the location of the tumor, the physiologic status of the patient, and the expertise available at the facility. It may include bronchoscopy, biopsy of palpable cervical or supraclavicular lymph nodes, needle biopsy of a lung mass or mediastinal nodes using either CT or ultrasound guidance, mediastinoscopy, mediastinotomy, median sternotomy, video-assisted thoracoscopy, and conventional thoracotomy. The biopsy findings will help the clinician plan appropriate treatment
History and Symptoms
The presentation depends on the degree of occlusion and the rapidity with which it develops. The most common symptoms are dyspnea, facial swelling/fullness (suffusion) and cough. The most common signs are venous distention of the neck and chest wall and facial edema. Less commonly, chest pain, dysphagia, proptosis, hemoptysis, glossal edema, hoarseness, and headache are described. Symptoms get worse with leaning forward, coughing or lying down. Typically symptoms are present for >3 months before diagnosis.
Physical Examination
Appearance of the Patient
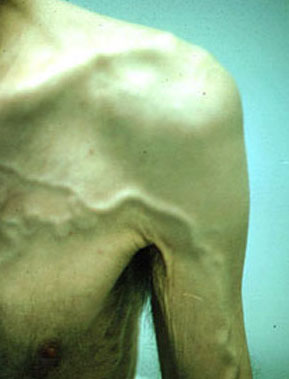
Pemberton’s Sign: suffusion, plethora, or duskiness that develop upon elevation of the arms above the head in patients with SVC syndrome.
Vital Signs
Skin
The skin of the face may have plethora.
There may be distension of veins on the torso.
Ear Nose and Throat
There is swelling of the face.
Laboratory Findings
Chest X Ray
This is a useful test to exclude lung cancer
MRI and CT
Useful in evaluating source and extent of a neoplasm.
Other Diagnostic Studies
Biopsy may be neccessary to evaluate the underlying cause.
Risk Stratification and Prognosis
SVC syndrome is rarely fatal, and the prognosis is generally related to the prognosis of the underlying malignancy rather than the presence/absence of venous obstruction.
Differential Diagnosis
The leading cancers associated with SVC syndrome are bronchogenic lung cancer (particularly small cell lung cancer), breast cancer, and lymphoma.
Benign causes of SVC syndrome include mediastinal fibrosis, histoplasmosis, radiation therapy complications, retrosternal goiter, Behcet’s syndrome, and thrombosis due to indwelling catheters or pacemakers. In the past, Tuberculosis and Syphilis were major players.
Treatment
The treatment of SVCS depends on the etiology of the obstruction, the severity of the symptoms, the prognosis of the patient, and patient preferences and goals for therapy. Radiation therapy or chemotherapy should be withheld until the etiology of the obstruction is clear. The treatments discussed here focus on SVC obstruction caused by a malignant tumor. Since the treatment of malignant obstruction may depend on tumor histology, a histologic diagnosis, if not made earlier, should be made prior to initiation of treatment. Unless there is airway obstruction or cerebral edema, there appears to be no detriment in outcome when treatment is delayed for the assessment. [2] [3] [4]
Medical Management
A patient with sufficient collateral blood flow and minimal symptoms may not need treatment. If the lesion is above the azygous vein or if the onset of SVC occlusion is slow enough to allow sufficient collateral circulation, the symptoms and signs may stabilize and the patient may be comfortable enough to forego further therapy. Short-term palliation of a symptomatic patient who does not want aggressive treatment may be achieved by elevating the head and using corticosteroids and diuresis. There are no definitive studies that prove the effectiveness of steroids, although they are potentially useful to treat respiratory compromise. Diuretics may give symptomatic relief of edema but can ultimately cause systemic complications, such as dehydration.
Radiation Therapy
If the obstruction of the SVC is caused by a tumor that is not sensitive to chemotherapy, radiation therapy should be given. Treatment with larger fractions of radiation is thought to be beneficial in developing a rapid response. One study shows, however, that there is no obvious need for large radiation fraction sizes for the first few radiation treatments as was previously believed. Many fractionation schemes have been used, with doses ranging from 30 Gy in 10 fractions to 50 Gy in 25 fractions. Relief of symptoms in small cell lung cancer is reported to be 62% to 80%, whereas in non-small cell lung cancer, approximately 46% of the patients experienced symptomatic relief. In one study, more than 90% of the patients achieved a partial or complete response with a 3-week regimen of 8 Gy given once a week for a total dose of 24 Gy.
Chemotherapy
Chemotherapy is the treatment of choice for sensitive tumors such as lymphoma or small cell lung cancer. SVCS does not appear to be an independent prognostic factor, and its presence should not change the treatment approach. Rapid initiation of chemotherapy can result in complete and partial response rates of the SVCS of more than 80% in small cell lung cancer patients.
Thrombolysis
It has been suggested that SVCS arises when a thrombus forms in a partially occluded vein. In patients with a documented thrombus in the SVC, treatment may include thrombectomy, with or without tissue plasminogen activator or other thrombolytic agents such as streptokinase or urokinase.
Stent Placement
There have been numerous small studies using an intravascular expandable stent to reopen the occluded SVC; however, no prospectively designed comparative studies have been published. The reported response rates have been about 90% or greater. There is no agreement on the need for anticoagulant therapy after stent placement. In one series that used anticoagulant therapy for patients as part of the treatment protocol, there were reports of reocclusion after this therapy was stopped. However, in another study, 17 cancer patients who were treated with stents and who did not have anticoagulant therapy had no occlusions.
Surgery
Surgical bypass of an obstructed SVC is more appropriate for patients with a benign obstruction than with a malignant obstruction, although surgical bypass has also been used for patients with malignant obstructions.
Psychosocial Considerations
Patients and family members are often frightened and anxious because of the symptoms produced by SVCS, particularly swelling, dysphagia, coughing, and hoarseness. It is important to provide information to patients and family members on the cause of the symptoms and on short-term measures for palliation, especially during the diagnostic period. When aggressive treatment is declined because of the terminal nature of the underlying disease, it may be necessary to teach symptom management approaches to patients and family members.
Because most adult patients who develop SVCS have lung cancer, the treatment and psychologic support approaches that are developed for SVCS should take into account the patient’s prognosis and psychologic condition and other symptoms caused by the malignancy.
Pediatric Considerations
SVCS refers to the symptoms associated with the compression or obstruction of the SVC; the compression of the trachea is termed superior mediastinal syndrome (SMS). Because SMS and the resulting respiratory compromise frequently occur in children with SVCS, the two syndromes have become almost synonymous in pediatric practice. In adults, the trachea and the right mainstem bronchus are relatively rigid structures compared with the vena cava, but in children these structures are more susceptible to compression. In addition, the relatively smaller intraluminal diameters of a child’s trachea and bronchus can tolerate little edema before respiratory symptoms occur. Because of this accompanying respiratory component, SVCS in children differs from the adult syndrome and constitutes a serious medical emergency.
The most common symptoms of SVCS in children are similar to those in adults and include coughing, hoarseness, dyspnea, orthopnea, and chest pain. Symptoms that are less common but more serious are anxiety, confusion, lethargy, headache, distorted vision, a sense of fullness in the ears, and, especially, syncope.
SVCS is rare in children and appears at presentation in 12% of pediatric patients with malignant mediastinal tumors. The etiology, diagnosis, and treatment of SVCS in children differs from that in adults. Whereas the most frequent cause of SVCS in adults is bronchogenic carcinoma, in children the most frequent malignant cause of the syndrome is non-Hodgkin lymphoma. As in adults, a frequent nonmalignant cause is thrombosis from catheterization for venous access.
A physical examination, chest radiograph, and the medical history of the patient are usually sufficient to establish a diagnosis of SVCS. If lymphoma or other malignant disease is suspected, it is desirable to obtain a tissue sample for diagnosis. However, the procedure to obtain the specimen may involve significant risk and may not be clinically feasible. Children with SVCS have a poor tolerance for the necessary general anesthesia because the accompanying cardiovascular and pulmonary changes aggravate the SVCS, often making intubation impossible. Also, extubation may be difficult or impossible, thus requiring prolonged airway provision (intubation). A CT scan of the chest to determine tracheal size, upright and supine echocardiography, and a flow volume loop may help evaluate anesthetic risk. Because anesthesia use is a serious risk, the diagnosis should be made with the least invasive means possible. Published reports suggest a stepwise approach to diagnosis.
When a malignant mass is the cause of the SVCS, the situation may be a medical emergency with no opportunity to establish a tissue diagnosis. In these cases, the most appropriate course may be to initiate empiric therapy prior to biopsy. The traditional empiric therapy is irradiation, with the daily dose governed by the presumed radiosensitivity of the tumor. After irradiation, respiratory deterioration from the apparent tracheal swelling may occur because of the inability of narrow lumens in children to accommodate edema and because of the greater degree of edema at onset, which is due to the rapid speed at which tumors grow in children. In these situations, a course of prednisone at 10 mg/m2 of body surface area 4 times per day may be necessary.
In addition to radiation, empiric therapy of SVCS has included chemotherapeutic agents incorporating steroids, cyclophosphamide, or both in combination with an anthracycline and vincristine. If the tumor fails to respond, it may be a benign lesion.
If surgery becomes necessary, it should be performed with the patient in the semi-Fowler’s position, allowing the surgeon the ability to rapidly change the patient's position to lateral or prone. Cardiopulmonary bypass facilities and a rigid bronchoscope should be available in a standby capacity.
References
- ↑ Beeson, Michael S. "Superior Vena Cava Syndrome". Retrieved 2008-03-24.
- ↑ Nieto AF, Doty DB. Superior vena cava obstruction: clinical syndrome, etiology, and treatment. Curr Probl Cancer 1986; 10:441-84.
- ↑ Yellin A, Rosen A, Reichert N, Lieberman Y. Superior vena cava syndrome. The myth--the facts. Am Rev Respir Dis 1990; 141:1114-8.
- ↑ Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990; 160:207-11.
See also
Acknowledgements
Source of Initial Content: Morning report notes prepared by Dr. Duane Pinto, C. Michael Gibson, M.S., M.D.