Reserpine
 | |
| Clinical data | |
|---|---|
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50% |
| Metabolism | gut/liver |
| Elimination half-life | phase 1 = 4.5h, phase 2 = 271h, average = 33h |
| Excretion | 62% feces / 8% urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
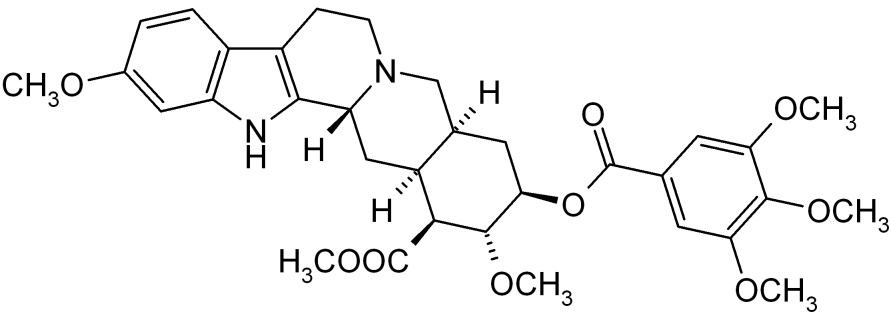
| Formula | C33H40N2O9 |
| Molar mass | 608.68 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [4] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
For patient information, click here
Overview
Reserpine is an indole alkaloid[5] antipsychotic and antihypertensive drug that has been used for the control of high blood pressure and for the relief of psychotic behaviors, although because of the development of better drugs for these purposes and because of its numerous side-effects, it is rarely used today.[1] The antihypertensive actions of Reserpine are a result of its ability to deplete catecholamines (among the others) from peripheral sympathetic nerve endings. These substances are normally involved in controlling heart rate, force of cardiac contraction and peripheral resistance. .[6] Reserpine depletion of monoamine neurotransmitters in the synapses is often cited as evidence to the theory that depletion of the neurotransmitters causes subsequent depression in humans. Moreover, reserpine has a peripheral action in many parts of the body, resulting in a preponderance of the cholinergic part of the nervous system (GI-Tract, smooth muscles vessels).
Mode of action
Reserpine acts via disruption of norepinepherine, serotonin, and dopamine presynaptic vesicles by the transporter VMAT. The neurotransmitters are subsequently metabolized by MAO and therefore never reach the synapse.
History
Reserpine was isolated in 1952 from the dried root of Rauwolfia serpentina (Indian snakeroot),[7] (which had been known as Sarpaganda and had been used for centuries there for the treatment of insanity, as well as fever and snakebites[2]) and introduced it in 1954, two years after chlorpromazine.[8] Reserpine almost irreversibly blocks the uptake (and storage) of norepinephrine (i.e. noradrenaline) and dopamine into synaptic vesicles by inhibiting the Vesicular Monoamine Transporters (VMAT).[9]
Reserpine has been discontinued in the UK for some years due to its vast interactions and side effects.
Reserpine was also highly influential in promoting the thought of a biogenic-amine hypothesis of depression - see Everett & Tolman, 1959.
Uses today
Reserpine is one of the few antihypertensive medications that have been shown in randomized controlled trials to reduce mortality: The Hypertension Detection and Follow-up Program,[3] the Veterans Administration Cooperative Study Group in Anti-hypertensive Agents,[4] and the Systolic Hypertension n the Elderly Program.[5]
Reserpine is listed as a second line choice by the JNC 7.[6] Reserpine is an excellent second agent for patients who are uncontrolled on a diuretic.[7]
In some countries reserpine is still available as part of combination drugs for the treatment of hypertension, in most cases they contain also a diuretic and/or a vasodilator like hydralazine. These combinations are currently regarded as second choice drugs. The daily dose of reserpine in antihypertensive treatment is as low as 0.1 to 0.25mg. The use of reserpine as an antipsychotic drug has been nearly completely abandoned. Originally, doses of 0.5mg to 40mg daily were used to treat psychotic diseases. Doses in excess of 3mg daily often required use of an anticholinergic drug to combat excessive cholinergic activity in many parts of the body as well as parkinsonism. Reserpine may be used as a sedative for horses.
Side effects
At doses of less than 0.2 mg/day, reserpine has few side effects, most commonly is nasal congestion.[8]
There has been much concern about reserpine causing depression leading to suicide. However, this was reported in uncontrolled studies using doses averaging 0.5 mg per day.[9][10]
Reserpine can cause: nasal congestion, nausea, vomiting, weight gain, gastric intolerance, gastric ulceration (due to increased cholinergic activity in gastric tissue and impaired mucosal quality), stomach cramps and diarrhea are noted. The drug causes hypotension and bradycardia and may worsen asthma. Congested nose and erectile dysfunction are other consequences of alpha-blockade. Depression can occur at any dose and may be severe enough to lead to suicide. Other central effects are a high incidence of drowsiness, dizziness, and nightmares. Parkinsonism occurs in a dose dependent manner. General weakness or fatigue is quite often encountered. High dose studies in rodents found reserpine to cause fibroadenoma of the breast and malignant tumors of the semen vesicles among others. Early suggestions that reserpine causes breast cancer in women (risk approximately doubled) were not confirmed. Besides, it may also cause hyperprolactinemia.
References
- ↑ [1] The Columbia Encyclopedia, Sixth Edition. Copyright © 2001-05 Columbia University Press.
- ↑ Op. cit. Columbia Encyclopedia
- ↑ "Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-up Program Cooperative Group". JAMA. 242 (23): 2562–71. 1979. PMID 490882. full text at OVID
- ↑ "Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg". JAMA. 202 (11): 1028–34. 1967. PMID 4862069.
- ↑ "Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group". JAMA. 265 (24): 3255–64. 1991. PMID 2046107.
- ↑ Chobanian AV, Bakris GL, Black HR; et al. (2003). "The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report". JAMA. 289 (19): 2560–72. doi:10.1001/jama.289.19.2560. PMID 12748199. summary
- ↑ Moser M (1987). ""Cost containment" in the management of hypertension". Ann. Intern. Med. 107 (1): 107–9. PMID 3592424.
- ↑ Curb JD, Schneider K, Taylor JO, Maxwell M, Shulman N (1988). "Antihypertensive drug side effects in the Hypertension Detection and Follow-up Program". Hypertension. 11 (3 Pt 2): II51–5. PMID 3350594.
- ↑ QUETSCH RM, ACHOR RW, LITIN EM, FAUCETT RL (1959). "Depressive reactions in hypertensive patients; a comparison of those treated with Rauwolfia and those receiving no specific antihypertensive treatment". Circulation. 19 (3): 366–75. PMID 13629798.
- ↑ LEMIEUX G, DAVIGNON A, GENEST J (1956). "Depressive states during Rauwolfia therapy for arterial hypertension; a report of 30 cases". Canadian Medical Association journal. 74 (7): 522–6. PMID 13304797.
Additional Resources
- ^ アルカロイド (Alkaloids) (T-Z). 2004.
- ^ "Indole Alkaloids" Major Types Of Chemical Compounds In Plants & Animals Part II: Phenolic Compounds, Glycosides & Alkaloids. Wayne's Word: An On-Line Textbook of Natural History. 2005.
- ^ Forney, Barbara. Reserpine for Veterinary Use Wedgewood Pharmacy. 2001-2002.
- ^ Rauwolfia Dorlands Medical Dictionary. Merck Source. 2002.
- ^ Lopez-Munoz F, Bhatara VS, Alamo C, Cuenca E. (2004): "[Historical approach to reserpine discovery and its introduction in psychiatry]" [Article in Spanish] Actas Esp Psiquiatr. 32(6):387-95. PMID 15529229 Fulltext in English and Spanish
- ^ Schuldiner, S. et al. (1993): J. Biol. Chem. 268(1) 29-34. PMID 8416935
External links
- NLM Hazardous Substances Databank – Reserpine
- PubChem Substance Summary: Reserpine National Center for Biotechnology Information.
- The Stork Synthesis of (-)-Reserpine
de:Reserpin
hr:Rezerpin
sk:Rezerpín
- Pages with script errors
- CS1 maint: Explicit use of et al.
- CS1 maint: Multiple names: authors list
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Indole alkaloids
- VMAT inhibitors
- Antihypertensive agents
- Drugs