Primary hyperaldosteronism pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| (10 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
<div style="-webkit-user-select: none;"> | <div style="-webkit-user-select: none;"> | ||
{|class="infobox" style="position: fixed; top: 65%; right: 10px; margin: 0 0 0 0; border: 0; float: right; | {| class="infobox" style="position: fixed; top: 65%; right: 10px; margin: 0 0 0 0; border: 0; float: right;" | ||
|- | |- | ||
| {{#ev:youtube|https://https://www.youtube.com/watch?v=JBfkGNr01V8|350}} | | {{#ev:youtube|https://https://www.youtube.com/watch?v=JBfkGNr01V8|350}} | ||
| Line 8: | Line 8: | ||
__NOTOC__ | __NOTOC__ | ||
{{Primary hyperaldosteronism}} | {{Primary hyperaldosteronism}} | ||
{{CMG}}; {{AE}} {{HK}}, {{MJ}} | |||
== Overview == | == Overview == | ||
Conn's syndrome (primary | Conn's syndrome (primary hyperaldosteronism) features overproduction of [[aldosterone]] despite suppressed [[Plasma renin activity|plasma renin activity (PRA)]]. The resulting [[sodium]] retention produces [[hypertension]], and elevated [[potassium]] excretion may cause [[hypokalemia]]. Patients with Conn's syndrome due to primary hyperaldosteronism may have an [[aldosterone]] producing adrenocortical adenoma (APA); classically referred to as Conn's syndrome, a unilateral hyperplasia, idiopathic hyperaldosteronism (IHA, also known as bilateral adrenal hyperplasia), familial forms (familial hyperaldosteronism types I, II, and III) have also been described, [[ectopic]] secretion of [[aldosterone]] (The [[Ovary|ovaries]] and [[kidneys]] are the 2 organs described in the literature that, in the setting of [[neoplastic disease]], can be [[ectopic]] sources of [[aldosterone]], but this is a rare occurrence). | ||
== Pathophysiology == | == Pathophysiology == | ||
=== Renin === | |||
* In kidney nephron, there is a specialized system called [[juxtaglomerular apparatus]], which is located in the [[afferent arteriole]] of [[glomerulus]]. | |||
* [[Juxtaglomerular apparatus]] synthesizes pro-[[renin]], and then later it converts into [[renin]] with mediation of a [[proteolytic enzyme]]. | |||
* [[Renin]] is stored in and then may be released from secretory [[granules]], in response to various factors. | |||
* [[Renin]] releasing starts a cascade of steps, the first step is the cleavage of the [[angiotensin]] I from [[angiotensinogen]] ([[renin]] substrate). | |||
* [[Angiotensinogen]] is an [[Alpha globulin|alpha-2-globulin]] that is produced mainly in [[liver]] and [[kidney]]<nowiki/>s. | |||
* The first step is the rate-limiting step of the [[Renin-angiotensin system|renin-angiotensin]] cascade. | |||
* Most important stimuli to [[renin]] secretion are: | |||
** [[Renal]] [[hypoperfusion]], due to [[hypotension]] or [[volume depletion]] | |||
** Increased [[sympathetic]] activity | |||
=== Basic physiology of aldosterone === | === Basic physiology of aldosterone === | ||
Circulating [[aldosterone]] is principally made in the [[zona glomerulosa]] of the [[adrenal cortex]] (outer layer of the cortex) by a cascade of [[enzyme]] steps leading to the conversion of [[cholesterol]] to [[aldosterone]]. | Circulating [[aldosterone]] is principally made in the [[zona glomerulosa]] of the [[adrenal cortex]] (outer layer of the cortex) by a cascade of [[enzyme]] steps leading to the conversion of [[cholesterol]] to [[aldosterone]]. | ||
* [[Aldosterone]]'s production is regulated at two critical [[enzyme]] steps: | * [[Aldosterone]]'s production is regulated at two critical [[enzyme]] steps: | ||
* | *# Early, in its biosynthetic pathway (the conversion of [[cholesterol]] to [[pregnenolone]] by [[cholesterol]] side chain cleavage enzyme) | ||
* | *# Late, the conversion of [[corticosterone]] to [[aldosterone]] by [[aldosterone synthase]] | ||
* A variety of factors modify [[aldosterone]] secretion--the most important are [[angiotensin II]] (Ag II), the end-product of the [[renin-angiotensin system]] (RAS), and [[potassium]]. However [[Adrenocorticotropic hormone|ACTH]], neural mediators, and [[Natriuretic peptides|natriuretic factors]] also play a role in the feedback mechanism. | |||
* [[Aldosterone]]'s classical [[epithelial]] effect is to increase the transport of [[sodium]] across the [[Cell (biology)|cell]] in exchange for [[potassium]] and [[hydrogen]] ions.<ref name="pmid15947886">{{cite journal |vauthors=Williams GH |title=Aldosterone biosynthesis, regulation, and classical mechanism of action |journal=Heart Fail Rev |volume=10 |issue=1 |pages=7–13 |year=2005 |pmid=15947886 |doi=10.1007/s10741-005-2343-3 |url=}}</ref> | |||
[[Image:Renin-angiotensin system in man shadow.png|600px|thumb|center|Renin angiotensin system - by Mikael Häggström, Via: Wikimedia.org<ref><https://commons.wikimedia.org/w/index.php?curid=8458370></ref>]] | |||
[[ | [[image:Adrenal Steroids.png|thumb|600px|center|frame|Adrenal steroid synthesis pathways in adrenal cortex and related enzymes, Via: Wikimedia.org<ref name="urlFile:Adrenal Steroids Pathways.svg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/wiki/File:Adrenal_Steroids_Pathways.svg|title=File:Adrenal Steroids Pathways.svg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] | ||
==Pathogenesis == | ===Pathogenesis === | ||
Primary | Primary hyperaldosteronism (PA) features overproduction of [[aldosterone]] despite suppressed plasma [[renin]] activity (PRA). The resulting [[sodium]] retention may lead to [[hypertension]], and elevated [[potassium]] excretion may cause [[hypokalemia]]. | ||
* Patients with primary | * Patients with primary hyperaldosteronism may have: | ||
**Aldosterone producing adrenocortical adenoma (APA) | **Aldosterone producing adrenocortical adenoma (APA): Classically referred to as Conn's syndrome.<ref name="pmid17492946">{{cite journal |vauthors=Young WF |title=Primary aldosteronism: renaissance of a syndrome |journal=Clin. Endocrinol. (Oxf) |volume=66 |issue=5 |pages=607–18 |year=2007 |pmid=17492946 |doi=10.1111/j.1365-2265.2007.02775.x |url=}}</ref> | ||
**Unilateral [[hyperplasia]] | **Unilateral [[hyperplasia]] | ||
**Idiopathic hyperaldosteronism (IHA, also known as [[bilateral adrenal hyperplasia]]).<ref name="pmid26252618">{{cite journal |vauthors=Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T |title=Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype |journal=Clin. Endocrinol. (Oxf) |volume=83 |issue=6 |pages=779–89 |year=2015 |pmid=26252618 |pmc=4995792 |doi=10.1111/cen.12873 |url=}}</ref> | **Idiopathic hyperaldosteronism (IHA, also known as [[bilateral adrenal hyperplasia]]).<ref name="pmid26252618">{{cite journal |vauthors=Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T |title=Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype |journal=Clin. Endocrinol. (Oxf) |volume=83 |issue=6 |pages=779–89 |year=2015 |pmid=26252618 |pmc=4995792 |doi=10.1111/cen.12873 |url=}}</ref> | ||
**Familial forms (familial hyperaldosteronism types I, II, and III) have also been described. | **Familial forms (familial hyperaldosteronism types I, II, and III) have also been described. | ||
**[[Ectopic]] secretion of [[aldosterone]] ( | **[[Ectopic]] secretion of [[aldosterone]] (the [[ovaries]] and [[kidneys]] are the 2 organs described in the literature that, in the setting of [[neoplastic disease]], can be [[ectopic]] sources of [[aldosterone]], but this is a rare occurrence.) | ||
==Genetics == | ==Genetics == | ||
'''<u>1. Aldosterone Producing Adenoma (APA)</u>''' | '''<u>1. Aldosterone Producing Adenoma (APA)</u>''' | ||
| Line 36: | Line 53: | ||
===== Somatic mutations ===== | ===== Somatic mutations ===== | ||
* Primary hyperaldosteronism producing aldosterone-producing adenomas (APAs) have [[mutations]] in [[Gene|genes]] encoding [[ion channels]]/pumps that change the [[intracellular]] [[calcium]] [[homeostasis]] and cause [[renin]]-independent [[aldosterone]] production through enhanced [[CYP11B2]] expression. Subcapsular aldosterone-producing cell clusters (APCCs) are [[CYP11B2]]-expressing clusters of cells that are found beneath the adrenal capsule but protrude into cortisol-producing [[Cell (biology)|cells]] that are negative for [[CYP11B2]] expression. | * Primary hyperaldosteronism producing aldosterone-producing adenomas (APAs) have [[mutations]] in [[Gene|genes]] encoding [[ion channels]]/pumps that change the [[intracellular]] [[calcium]] [[homeostasis]] and cause [[renin]]-independent [[aldosterone]] production through enhanced [[CYP11B2]] expression. Subcapsular [[aldosterone]]-producing cell clusters (APCCs) are [[CYP11B2]]-expressing clusters of cells that are found beneath the adrenal capsule but protrude into cortisol-producing [[Cell (biology)|cells]] that are negative for [[CYP11B2]] expression. | ||
* APCCs are also frequently found in [[Adrenal gland|adrenal]] [[Tissue (biology)|tissue]] in close proximity to APA. | * APCCs are also frequently found in [[Adrenal gland|adrenal]] [[Tissue (biology)|tissue]] in close proximity to APA. | ||
* The [[Renin-angiotensin system|renin-angiotensin axis]] is supressed in patients with APAs, pointing towards an autonomous, [[renin]]-independent production of [[aldosterone]] by APCCs. | * The [[Renin-angiotensin system|renin-angiotensin axis]] is supressed in patients with APAs, pointing towards an autonomous, [[renin]]-independent production of [[aldosterone]] by APCCs. | ||
* [[Somatic mutation|Somatic mutations]] in ''KCNJ5'', ''ATP1A1'', ''ATP2B3'', and ''CACNA1D'' are found in approximately 50 percent of | * [[Somatic mutation|Somatic mutations]] in ''KCNJ5'', ''ATP1A1'', ''ATP2B3'', and ''CACNA1D'' are found in approximately 50 percent of APAs. | ||
=====Gain of function mutations(KCNJ5, CACNA1D, CTNNB1 mutations)===== | =====Gain of function mutations (KCNJ5, CACNA1D, CTNNB1 mutations)===== | ||
* [[Inherited]] and [[Acquired disorder|acquired mutations]] | * [[Inherited]] and [[Acquired disorder|acquired mutations]] in [[potassium]] inwardly rectifying channel, subfamily J, member 5 (''[[KCNJ5]])'' [[gene]], which codes for a K ion [[Ion channel|channel]] has been associated with autonomous [[cell proliferation]] in the [[adrenal cortex]].<ref name="pmid21311022">{{cite journal |vauthors=Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RP |title=K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension |journal=Science |volume=331 |issue=6018 |pages=768–72 |year=2011 |pmid=21311022 |pmc=3371087 |doi=10.1126/science.1198785 |url=}}</ref> Two somatic [[mutations]] in the K<sup>+</sup> channel [[KCNJ5]] (G151R and L168R) cause ~40% of APA.<ref name="pmid262526182">{{cite journal |vauthors=Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T |title=Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype |journal=Clin. Endocrinol. (Oxf) |volume=83 |issue=6 |pages=779–89 |year=2015 |pmid=26252618 |pmc=4995792 |doi=10.1111/cen.12873 |url=}}</ref> These [[mutations]] affect [[K]] ion selectivity leading to increased [[Sodium|Na]] conductance and [[Depolarization|membrane depolarization]] resulting in activation of [[Voltage-gated ion channel|voltage-gated Ca2+channels]]. Increased [[intracellular]] Ca results in [[CYP11B2]] expression and release of [[aldosterone]] from the [[adrenal gland]]. Females are more prone to [[KCNJ5]] [[mutations]]. These group of gene mutations occur in younger people and these patients have higher minimal [[Blood plasma|plasma]] [[potassium]] concentrations.<ref name="pmid24866132">{{cite journal |vauthors=Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, Mantero F, Cicala MV, Quinkler M, Fallo F, Allolio B, Bernini G, Maccario M, Giacchetti G, Jeunemaitre X, Mulatero P, Reincke M, Zennaro MC |title=Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma |journal=Hypertension |volume=64 |issue=2 |pages=354–61 |year=2014 |pmid=24866132 |doi=10.1161/HYPERTENSIONAHA.114.03419 |url=}}</ref><ref name="pmid22628608">{{cite journal |vauthors=Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV, Pezzani R, Auchus RJ, Ghayee HK, Shibata H, Kurihara I, Williams TA, Giri JG, Bollag RJ, Edwards MA, Isales CM, Rainey WE |title=Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells |journal=J. Clin. Endocrinol. Metab. |volume=97 |issue=8 |pages=E1567–72 |year=2012 |pmid=22628608 |pmc=3410264 |doi=10.1210/jc.2011-3132 |url=}}</ref><ref name="pmid24037882">{{cite journal |vauthors=Monticone S, Hattangady NG, Penton D, Isales CM, Edwards MA, Williams TA, Sterner C, Warth R, Mulatero P, Rainey WE |title=a Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III |journal=J. Clin. Endocrinol. Metab. |volume=98 |issue=11 |pages=E1861–5 |year=2013 |pmid=24037882 |pmc=3816265 |doi=10.1210/jc.2013-2428 |url=}}</ref><ref name="pmid22315453">{{cite journal |vauthors=Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE |title=Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis |journal=Endocrinology |volume=153 |issue=4 |pages=1774–82 |year=2012 |pmid=22315453 |pmc=3320257 |doi=10.1210/en.2011-1733 |url=}}</ref> | ||
* A [[germline mutation]] in the [[KCNJ5]] gene produces familial hyperaldosteronism type III. | * A [[germline mutation]] in the [[KCNJ5]] gene produces familial hyperaldosteronism type III. | ||
*Gain-of-function [[mutation]] in the ''CACNA1D [[gene]] | *Gain-of-function [[mutation]] in the ''CACNA1D [[gene]]:'' CACNA1D [[mutation]] leads to increased [[calcium]] influx through the mutant [[Ion channel|channel]] by shifting the voltage dependence of activation to less [[Depolarization|depolarized]] potentials and, in some cases, impairing inactivation. | ||
*Activating somatic CTNNB1 [[mutations]], which mediate their effects through WnT signalling pathway have also been known to cause APA.<ref name="pmid26815163">{{cite journal |vauthors=Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P |title=Activating mutations in CTNNB1 in aldosterone producing adenomas |journal=Sci Rep |volume=6 |issue= |pages=19546 |year=2016 |pmid=26815163 |pmc=4728393 |doi=10.1038/srep19546 |url=}}</ref> | *Activating somatic CTNNB1 [[mutations]], which mediate their effects through WnT signalling pathway have also been known to cause APA.<ref name="pmid26815163">{{cite journal |vauthors=Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P |title=Activating mutations in CTNNB1 in aldosterone producing adenomas |journal=Sci Rep |volume=6 |issue= |pages=19546 |year=2016 |pmid=26815163 |pmc=4728393 |doi=10.1038/srep19546 |url=}}</ref> | ||
*CTNNB1 [[Mutation|mutations]] cause [[Adrenal cortex|adrenocortical cells]] to | *CTNNB1 [[Mutation|mutations]] cause [[Adrenal cortex|adrenocortical cells]] to dedifferentiate into their the precursor [[Adrenal gland|adrenal]] gonadal cell. | ||
==== Loss of function mutations(ATP1A1 and ATP2A3) ==== | ==== Loss of function mutations (ATP1A1 and ATP2A3) ==== | ||
* Other genes implicated in development of APAs are loss-of-function [[mutations]] in [[ATP1A1]] and [[ATP2A3]] [[Gene|genes]]. | * Other genes implicated in development of APAs are loss-of-function [[mutations]] in [[ATP1A1]] and [[ATP2A3]] [[Gene|genes]]. | ||
*[[ATP1A1]] [[Mutation|mutations]] lead to permeability of the pump for Na+ or H+ ions in a channel-like mode, again causing depolarization and release of [[aldosterone]].<ref name="pmid262526183">{{cite journal |vauthors=Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T |title=Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype |journal=Clin. Endocrinol. (Oxf) |volume=83 |issue=6 |pages=779–89 |year=2015 |pmid=26252618 |pmc=4995792 |doi=10.1111/cen.12873 |url=}}</ref> | *[[ATP1A1]] [[Mutation|mutations]] lead to permeability of the pump for Na+ or H+ ions in a channel-like mode, again causing depolarization and release of [[aldosterone]].<ref name="pmid262526183">{{cite journal |vauthors=Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T |title=Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype |journal=Clin. Endocrinol. (Oxf) |volume=83 |issue=6 |pages=779–89 |year=2015 |pmid=26252618 |pmc=4995792 |doi=10.1111/cen.12873 |url=}}</ref> | ||
| Line 59: | Line 76: | ||
'''<u>4. Familial hyperaldosteronism Type III</u>''' | '''<u>4. Familial hyperaldosteronism Type III</u>''' | ||
*FH-III has been known to be caused due to mutation in the [[KCNJ5]] [[gene]].<ref name="urlGenetics of primary hyperaldosteronism">{{cite web |url=http://erc.endocrinology-journals.org/content/23/10/R437.long |title=Genetics of primary hyperaldosteronism |author= |authorlink= |coauthors= |date= |format= |work= |publisher= |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=}}</ref> | *FH-III has been known to be caused due to mutation in the [[KCNJ5]] [[gene]].<ref name="urlGenetics of primary hyperaldosteronism">{{cite web |url=http://erc.endocrinology-journals.org/content/23/10/R437.long |title=Genetics of primary hyperaldosteronism |author= |authorlink= |coauthors= |date= |format= |work= |publisher= |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=}}</ref> | ||
*The [[tyrosine]]-to-[[cysteine]] substitution leads to increased Na | *The [[tyrosine]]-to-[[cysteine]] substitution leads to increased [[Sodium|Na]] permeability, [[Depolarization|cell membrane depolarization]], and disturbed intracellular [[Calcium|Ca]] homeostasis.<ref name="pmid24037882">{{cite journal |vauthors=Monticone S, Hattangady NG, Penton D, Isales CM, Edwards MA, Williams TA, Sterner C, Warth R, Mulatero P, Rainey WE |title=a Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III |journal=J. Clin. Endocrinol. Metab. |volume=98 |issue=11 |pages=E1861–5 |year=2013 |pmid=24037882 |pmc=3816265 |doi=10.1210/jc.2013-2428 |url= |issn=}}</ref> | ||
== Associated Conditions == | == Associated Conditions == | ||
| Line 70: | Line 87: | ||
==Gross Pathology== | ==Gross Pathology== | ||
* An [[aldosterone]] producing adenoma is usually, a unilateral, yellow, [[lipid]]-laden [[adenoma]] ranging in diameter from 5 to 35 mm. | * An [[aldosterone]] producing adenoma is usually, a unilateral, yellow, [[lipid]]-laden [[adenoma]] ranging in diameter from 5 to 35 mm. | ||
[[Image:Gross_adrenal_adenoma.jpeg| | <figure-inline>[[Image:Gross_adrenal_adenoma.jpeg|200x200px]]</figure-inline> | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
Microscopically, on [[Hematoxylin and eosin stain|hematoxylin and eosin]] section the following findings can be observed for [[aldosterone]] producing [[Adenoma|adenomas]]:<ref name="urlAldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism">{{cite web |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2889888/ |title=Aldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism |author= |authorlink= |coauthors= |date= |format= |work= |publisher= |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=}}</ref> | Microscopically, on [[Hematoxylin and eosin stain|hematoxylin and eosin]] section, the following findings can be observed for [[aldosterone]] producing [[Adenoma|adenomas]]:<ref name="urlAldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism">{{cite web |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2889888/ |title=Aldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism |author= |authorlink= |coauthors= |date= |format= |work= |publisher= |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=}}</ref> | ||
* The [[tumor]] usually consists of [[zona fasciculata]]-type [[Cell (biology)|cells]] although [[zona glomerulosa]]- or mixed cell-type tumors have been described. | * The [[tumor]] usually consists of [[zona fasciculata]]-type [[Cell (biology)|cells]] although [[zona glomerulosa]]- or mixed cell-type [[tumors]] have been described. | ||
* [[Aldosterone]]-secreting [[Adrenal carcinoma|adrenal carcinomas]] are very rare. These [[malignant]] [[Tumor|tumors]] exceed 40 mm in size with invasion of local [[Lymph node|lymph nodes]] or invasion of adjacent organs. | * [[Aldosterone]]-secreting [[Adrenal carcinoma|adrenal carcinomas]] are very rare. These [[malignant]] [[Tumor|tumors]] exceed 40 mm in size with invasion of local [[Lymph node|lymph nodes]] or invasion of adjacent organs. | ||
[[Image:Adenoma_adrenal.jpg| | <figure-inline>[[Image:Adenoma_adrenal.jpg|700x700px]]</figure-inline> | ||
Latest revision as of 15:12, 3 November 2017
| https://https://www.youtube.com/watch?v=JBfkGNr01V8%7C350}} |
|
Primary hyperaldosteronism Microchapters |
|
Differentiating Primary Hyperaldosteronism from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Primary hyperaldosteronism pathophysiology On the Web |
|
American Roentgen Ray Society Images of Primary hyperaldosteronism pathophysiology |
|
Risk calculators and risk factors for Primary hyperaldosteronism pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [2], Mehrian Jafarizade, M.D [3]
Overview
Conn's syndrome (primary hyperaldosteronism) features overproduction of aldosterone despite suppressed plasma renin activity (PRA). The resulting sodium retention produces hypertension, and elevated potassium excretion may cause hypokalemia. Patients with Conn's syndrome due to primary hyperaldosteronism may have an aldosterone producing adrenocortical adenoma (APA); classically referred to as Conn's syndrome, a unilateral hyperplasia, idiopathic hyperaldosteronism (IHA, also known as bilateral adrenal hyperplasia), familial forms (familial hyperaldosteronism types I, II, and III) have also been described, ectopic secretion of aldosterone (The ovaries and kidneys are the 2 organs described in the literature that, in the setting of neoplastic disease, can be ectopic sources of aldosterone, but this is a rare occurrence).
Pathophysiology
Renin
- In kidney nephron, there is a specialized system called juxtaglomerular apparatus, which is located in the afferent arteriole of glomerulus.
- Juxtaglomerular apparatus synthesizes pro-renin, and then later it converts into renin with mediation of a proteolytic enzyme.
- Renin is stored in and then may be released from secretory granules, in response to various factors.
- Renin releasing starts a cascade of steps, the first step is the cleavage of the angiotensin I from angiotensinogen (renin substrate).
- Angiotensinogen is an alpha-2-globulin that is produced mainly in liver and kidneys.
- The first step is the rate-limiting step of the renin-angiotensin cascade.
- Most important stimuli to renin secretion are:
- Renal hypoperfusion, due to hypotension or volume depletion
- Increased sympathetic activity
Basic physiology of aldosterone
Circulating aldosterone is principally made in the zona glomerulosa of the adrenal cortex (outer layer of the cortex) by a cascade of enzyme steps leading to the conversion of cholesterol to aldosterone.
- Aldosterone's production is regulated at two critical enzyme steps:
- Early, in its biosynthetic pathway (the conversion of cholesterol to pregnenolone by cholesterol side chain cleavage enzyme)
- Late, the conversion of corticosterone to aldosterone by aldosterone synthase
- A variety of factors modify aldosterone secretion--the most important are angiotensin II (Ag II), the end-product of the renin-angiotensin system (RAS), and potassium. However ACTH, neural mediators, and natriuretic factors also play a role in the feedback mechanism.
- Aldosterone's classical epithelial effect is to increase the transport of sodium across the cell in exchange for potassium and hydrogen ions.[1]
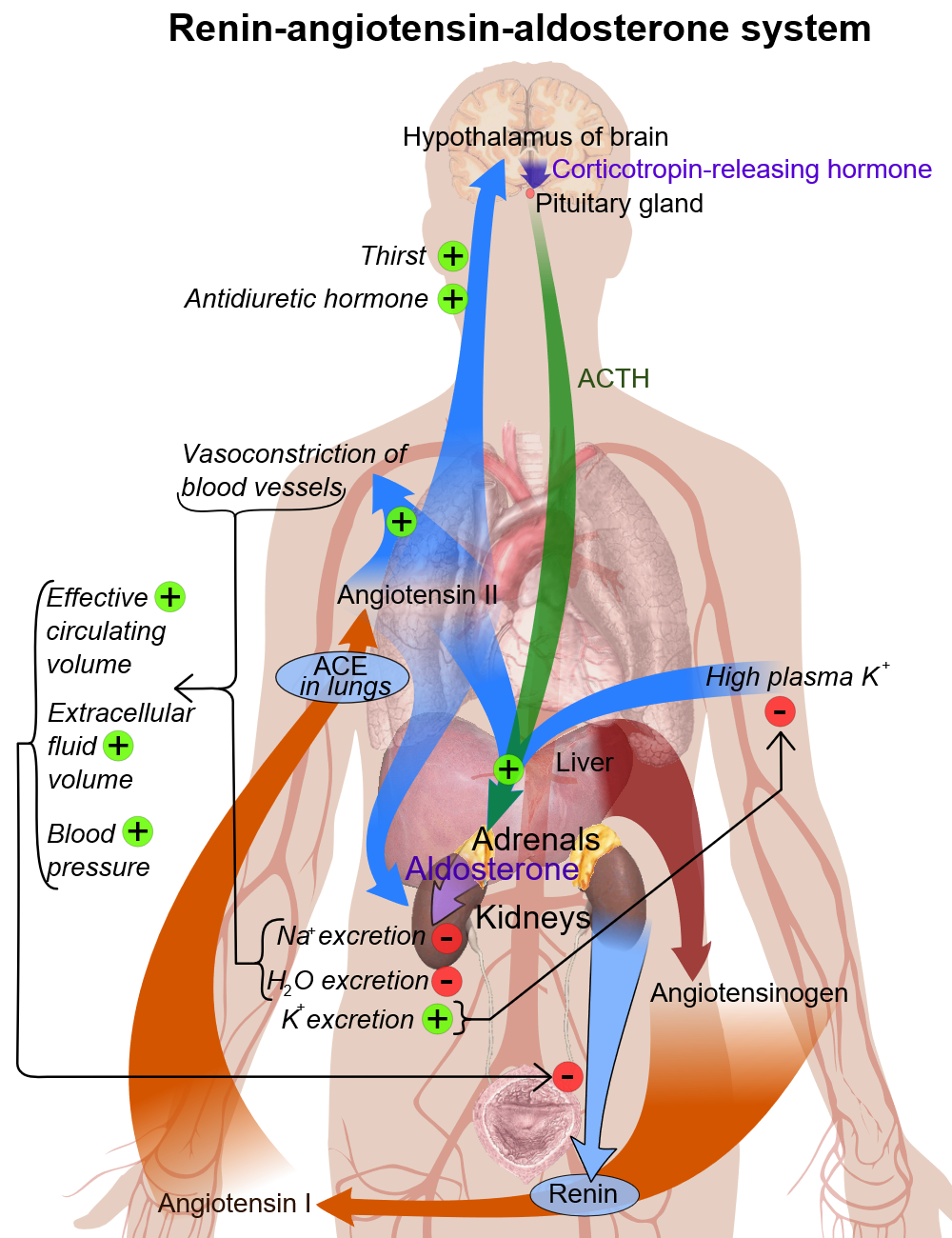
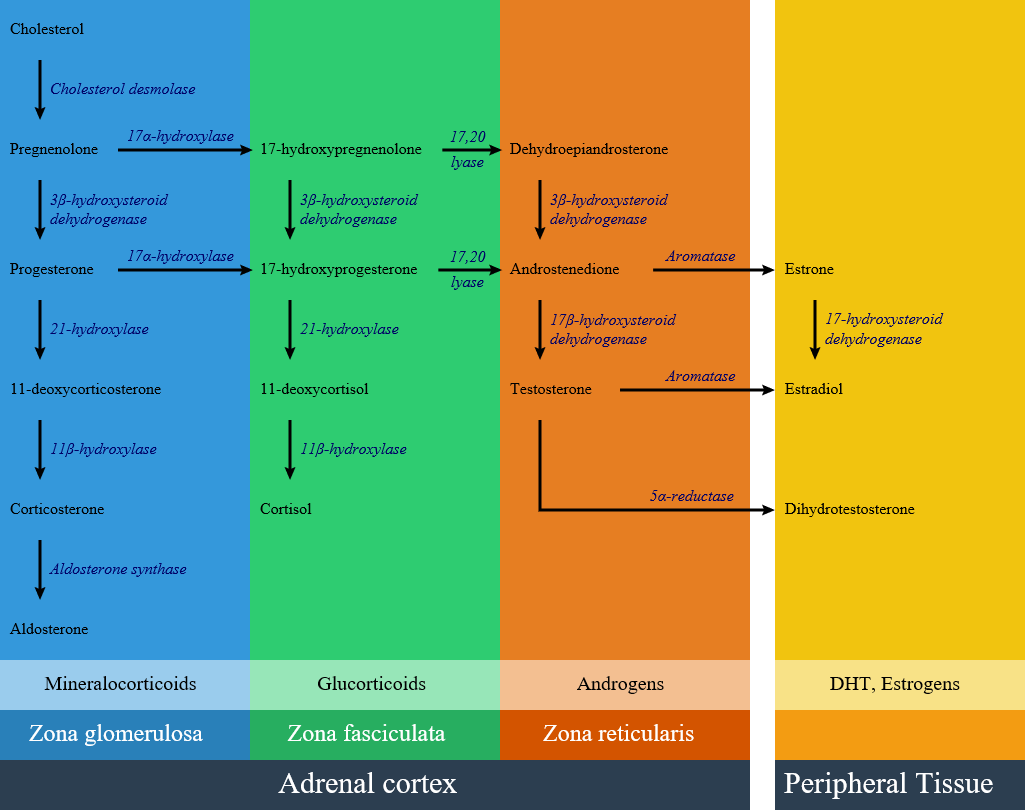
Pathogenesis
Primary hyperaldosteronism (PA) features overproduction of aldosterone despite suppressed plasma renin activity (PRA). The resulting sodium retention may lead to hypertension, and elevated potassium excretion may cause hypokalemia.
- Patients with primary hyperaldosteronism may have:
- Aldosterone producing adrenocortical adenoma (APA): Classically referred to as Conn's syndrome.[4]
- Unilateral hyperplasia
- Idiopathic hyperaldosteronism (IHA, also known as bilateral adrenal hyperplasia).[5]
- Familial forms (familial hyperaldosteronism types I, II, and III) have also been described.
- Ectopic secretion of aldosterone (the ovaries and kidneys are the 2 organs described in the literature that, in the setting of neoplastic disease, can be ectopic sources of aldosterone, but this is a rare occurrence.)
Genetics
1. Aldosterone Producing Adenoma (APA)
APAs are typically solitary, well circumscribed tumors which can cause aldosterone hypersecretion.
Somatic mutations
- Primary hyperaldosteronism producing aldosterone-producing adenomas (APAs) have mutations in genes encoding ion channels/pumps that change the intracellular calcium homeostasis and cause renin-independent aldosterone production through enhanced CYP11B2 expression. Subcapsular aldosterone-producing cell clusters (APCCs) are CYP11B2-expressing clusters of cells that are found beneath the adrenal capsule but protrude into cortisol-producing cells that are negative for CYP11B2 expression.
- APCCs are also frequently found in adrenal tissue in close proximity to APA.
- The renin-angiotensin axis is supressed in patients with APAs, pointing towards an autonomous, renin-independent production of aldosterone by APCCs.
- Somatic mutations in KCNJ5, ATP1A1, ATP2B3, and CACNA1D are found in approximately 50 percent of APAs.
Gain of function mutations (KCNJ5, CACNA1D, CTNNB1 mutations)
- Inherited and acquired mutations in potassium inwardly rectifying channel, subfamily J, member 5 (KCNJ5) gene, which codes for a K ion channel has been associated with autonomous cell proliferation in the adrenal cortex.[6] Two somatic mutations in the K+ channel KCNJ5 (G151R and L168R) cause ~40% of APA.[7] These mutations affect K ion selectivity leading to increased Na conductance and membrane depolarization resulting in activation of voltage-gated Ca2+channels. Increased intracellular Ca results in CYP11B2 expression and release of aldosterone from the adrenal gland. Females are more prone to KCNJ5 mutations. These group of gene mutations occur in younger people and these patients have higher minimal plasma potassium concentrations.[8][9][10][11]
- A germline mutation in the KCNJ5 gene produces familial hyperaldosteronism type III.
- Gain-of-function mutation in the CACNA1D gene: CACNA1D mutation leads to increased calcium influx through the mutant channel by shifting the voltage dependence of activation to less depolarized potentials and, in some cases, impairing inactivation.
- Activating somatic CTNNB1 mutations, which mediate their effects through WnT signalling pathway have also been known to cause APA.[12]
- CTNNB1 mutations cause adrenocortical cells to dedifferentiate into their the precursor adrenal gonadal cell.
Loss of function mutations (ATP1A1 and ATP2A3)
- Other genes implicated in development of APAs are loss-of-function mutations in ATP1A1 and ATP2A3 genes.
- ATP1A1 mutations lead to permeability of the pump for Na+ or H+ ions in a channel-like mode, again causing depolarization and release of aldosterone.[13]
2. Familial hyperaldosteronism Type I (FH-I)
- FH-I follows an autosomal dominant inheritance pattern.[14][15]
- Patients with FH-I inherit a chimeric CYP11B1 and CYP11B2 hybrid gene.[14]
3. Familial hyperaldosteronism Type II (FH-II)
- FH-II has an autosomal dominant inheritance.[15]
- It is caused due to germline mutations on a locus on chromosome 7 (specifically chromosome 7p22).[16][17]
4. Familial hyperaldosteronism Type III
- FH-III has been known to be caused due to mutation in the KCNJ5 gene.[18]
- The tyrosine-to-cysteine substitution leads to increased Na permeability, cell membrane depolarization, and disturbed intracellular Ca homeostasis.[10]
Associated Conditions
The following conditions may be found in association with primary hyperaldosteronism:
Gross Pathology
- An aldosterone producing adenoma is usually, a unilateral, yellow, lipid-laden adenoma ranging in diameter from 5 to 35 mm.
<figure-inline>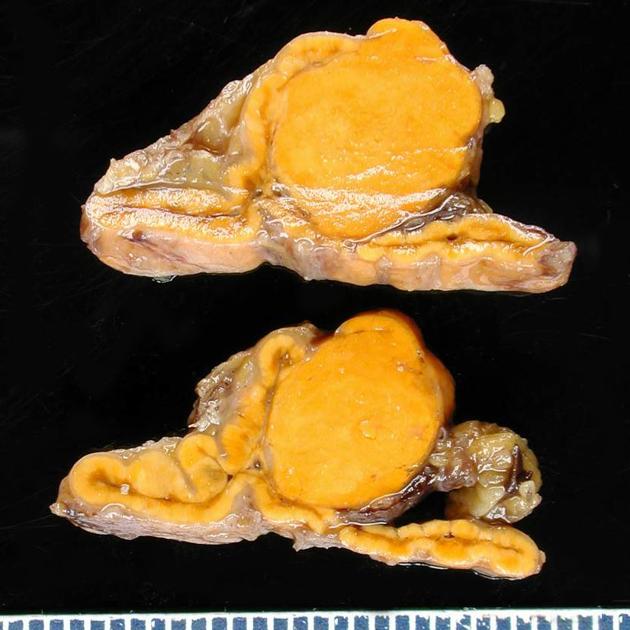 </figure-inline>
</figure-inline>
Microscopic Pathology
Microscopically, on hematoxylin and eosin section, the following findings can be observed for aldosterone producing adenomas:[23]
- The tumor usually consists of zona fasciculata-type cells although zona glomerulosa- or mixed cell-type tumors have been described.
- Aldosterone-secreting adrenal carcinomas are very rare. These malignant tumors exceed 40 mm in size with invasion of local lymph nodes or invasion of adjacent organs.
<figure-inline>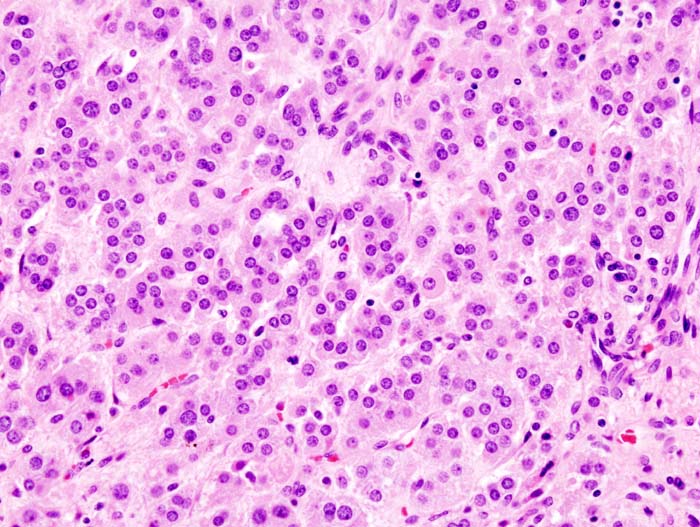 </figure-inline>
</figure-inline>
References
- ↑ Williams GH (2005). "Aldosterone biosynthesis, regulation, and classical mechanism of action". Heart Fail Rev. 10 (1): 7–13. doi:10.1007/s10741-005-2343-3. PMID 15947886.
- ↑ <https://commons.wikimedia.org/w/index.php?curid=8458370>
- ↑ "File:Adrenal Steroids Pathways.svg - Wikimedia Commons".
- ↑ Young WF (2007). "Primary aldosteronism: renaissance of a syndrome". Clin. Endocrinol. (Oxf). 66 (5): 607–18. doi:10.1111/j.1365-2265.2007.02775.x. PMID 17492946.
- ↑ Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T (2015). "Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype". Clin. Endocrinol. (Oxf). 83 (6): 779–89. doi:10.1111/cen.12873. PMC 4995792. PMID 26252618.
- ↑ Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RP (2011). "K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension". Science. 331 (6018): 768–72. doi:10.1126/science.1198785. PMC 3371087. PMID 21311022.
- ↑ Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T (2015). "Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype". Clin. Endocrinol. (Oxf). 83 (6): 779–89. doi:10.1111/cen.12873. PMC 4995792. PMID 26252618.
- ↑ Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, Mantero F, Cicala MV, Quinkler M, Fallo F, Allolio B, Bernini G, Maccario M, Giacchetti G, Jeunemaitre X, Mulatero P, Reincke M, Zennaro MC (2014). "Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma". Hypertension. 64 (2): 354–61. doi:10.1161/HYPERTENSIONAHA.114.03419. PMID 24866132.
- ↑ Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV, Pezzani R, Auchus RJ, Ghayee HK, Shibata H, Kurihara I, Williams TA, Giri JG, Bollag RJ, Edwards MA, Isales CM, Rainey WE (2012). "Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells". J. Clin. Endocrinol. Metab. 97 (8): E1567–72. doi:10.1210/jc.2011-3132. PMC 3410264. PMID 22628608.
- ↑ 10.0 10.1 Monticone S, Hattangady NG, Penton D, Isales CM, Edwards MA, Williams TA, Sterner C, Warth R, Mulatero P, Rainey WE (2013). "a Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III". J. Clin. Endocrinol. Metab. 98 (11): E1861–5. doi:10.1210/jc.2013-2428. PMC 3816265. PMID 24037882.
- ↑ Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE (2012). "Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis". Endocrinology. 153 (4): 1774–82. doi:10.1210/en.2011-1733. PMC 3320257. PMID 22315453.
- ↑ Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P (2016). "Activating mutations in CTNNB1 in aldosterone producing adenomas". Sci Rep. 6: 19546. doi:10.1038/srep19546. PMC 4728393. PMID 26815163.
- ↑ Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T (2015). "Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype". Clin. Endocrinol. (Oxf). 83 (6): 779–89. doi:10.1111/cen.12873. PMC 4995792. PMID 26252618.
- ↑ 14.0 14.1 Stowasser M, Gordon RD (2001). "Familial hyperaldosteronism". J. Steroid Biochem. Mol. Biol. 78 (3): 215–29. PMID 11595502.
- ↑ 15.0 15.1 Jackson RV, Lafferty A, Torpy DJ, Stratakis C (2002). "New genetic insights in familial hyperaldosteronism". Ann. N. Y. Acad. Sci. 970: 77–88. PMID 12381543.
- ↑ Lafferty AR, Torpy DJ, Stowasser M, Taymans SE, Lin JP, Huggard P, Gordon RD, Stratakis CA (2000). "A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22)". J. Med. Genet. 37 (11): 831–5. PMC 1734468. PMID 11073536.
- ↑ Torpy DJ, Gordon RD, Lin JP, Huggard PR, Taymans SE, Stowasser M, Chrousos GP, Stratakis CA (1998). "Familial hyperaldosteronism type II: description of a large kindred and exclusion of the aldosterone synthase (CYP11B2) gene". J. Clin. Endocrinol. Metab. 83 (9): 3214–8. doi:10.1210/jcem.83.9.5086. PMID 9745430.
- ↑ "Genetics of primary hyperaldosteronism".
- ↑ Malinow KC, Lion JR (1979). "Hyperaldosteronism (Conn's disease) presenting as depression". J Clin Psychiatry. 40 (8): 358–9. PMID 468762.
- ↑ Apostolopoulou K, Künzel HE, Gerum S, Merkle K, Schulz S, Fischer E, Pallauf A, Brand V, Bidlingmaier M, Endres S, Beuschlein F, Reincke M (2014). "Gender differences in anxiety and depressive symptoms in patients with primary hyperaldosteronism: a cross-sectional study". World J. Biol. Psychiatry. 15 (1): 26–35. doi:10.3109/15622975.2012.665480. PMID 22568586.
- ↑ Astegiano M, Bresso F, Demarchi B, Sapone N, Novero D, Palestro G, Resegotti A, Pellicano R, Rizzetto M (2005). "Association between Crohn's disease and Conn's syndrome. A report of two cases". Panminerva Med. 47 (1): 61–4. PMID 15985978.
- ↑ Kim YA, Lee SS (2003). "Conn's syndrome associated with Behcet's disease". J. Korean Med. Sci. 18 (1): 145–7. doi:10.3346/jkms.2003.18.1.145. PMC 3054990. PMID 12589107.
- ↑ "Aldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism".