Polycythemia pathophysiology: Difference between revisions
| (27 intermediate revisions by 3 users not shown) | |||
| Line 3: | Line 3: | ||
{{CMG}}{{AE}}{{Debduti}} | {{CMG}}{{AE}}{{Debduti}} | ||
==Overview== | ==Overview== | ||
*The main mechanism is a [[valine]] to [[phenylalanine]] substitution, particularly the [[JAK2]]V617F [[mutation]] which leads to increased red blood cells. Other mechanisms include [[Platelet|platelets]], [[Endothelium|endothelial]] compartment, [[leukocytes]], and [[plasma]] factors. | |||
==Pathophysiology== | |||
===Physiology=== | |||
*[[Polycythemia]] is considered a diagnosis when [[hematocrit]] is >48% in women and >52% in men, and when [[hemoglobin]] is >16.5g/dL in women and >18.5g/dL in men. <ref name="urlPolycythemia Symptoms, Causes, Treatment & Diagnosis">{{cite web |url=https://www.medicinenet.com/polycythemia_high_red_blood_cell_count/article.htm |title=Polycythemia Symptoms, Causes, Treatment & Diagnosis |format= |work= |accessdate=}}</ref> | |||
*It is important that we know the similarities and differences between [[polycythemia]] and [[erythrocytosis]]. | |||
*Similarities : | |||
Both are characterized by an increase in [[red blood cells]] in the blood. [[Genetics]] may play a role in both disorders. | |||
*Differences : | |||
[[Polycythemia]] is the increase in [[Red blood cell|red blood cells]] and [[hemoglobin]] above normal. [[Erythrocytosis]] is the increase in the mass of [[Red blood cell|red blood cells]]. [[Polycythemia]] may show an increase in [[white blood cells]] and [[Platelet|platelets]] as well. Increase in mass is limited to red blood cells only. <ref name="urlDifference Between Polycythemia and Erythrocytosis | Doctor HQ">{{cite web |url=https://doctorhq.com/index.php/2019/03/04/difference-between-polycythemia-and-erythrocytosis/#:~:text=The%20key%20difference%20between%20polycythemia%20and%20erythrocytosis%20is,blood%20cell%20mass%20increases%20beyond%20the%20normal%20level. |title=Difference Between Polycythemia and Erythrocytosis | Doctor HQ |format= |work= |accessdate=}}</ref> | |||
===Pathogenesis=== | |||
*The main mechanism by which [[polycythemia vera]] develops is a [[valine]] to [[phenylalanine]] substitution, precisely the JAK2V617F leading to constitutive activation of [[Cytokine receptor|cytokine]] receptors. This mutation is present in over 90% of patients with PV, 50% to 60% in patients with [[primary myelofibrosis]], and 50% with patients with [[essential thrombocythemia]].<ref name="pmid324915922">{{cite journal| author=| title=StatPearls | journal= | year= 2021 | volume= | issue= | pages= | pmid=32491592 | doi= | pmc= | url= }}</ref> | |||
*Other factors contributing to the [[pathophysiology]] of [[polycythemia]] (amongst other [[Myeloproliferative neoplasm|myeloproliferative]] neoplasms) are [[Blood cell|blood cells]], [[plasma]] factors, and the [[endothelial]] compartment. | |||
*'''The role of platelets:''' | |||
- Several studies in people and in mouse models have shown the increase in [[platelet]] activation and [[coagulation]] by factors such as cell surface [[Protein|proteins]] namely; [[P-selectin]] (CD62P), or [[tissue factor]](CD142), and circulating leuco-[[Platelet|platelets]] aggregates. | |||
An increase in [[CD40 (protein)|CD40]] [[ligand]], beta-thromboglobulin, [[platelet factor 4]], [[Thromboxane A2|thromboxane A]]2, and an increased expression of surface [[phosphatidylserine]] has been noted. | |||
*'''The role of leukocytes:''' | |||
- An increased expression of [[CD11]], [[CD14]], and [[leukocyte alkaline phosphatase]], which is further amplified in the event of a JAK2V617F mutation. The mutated macrophages also produce pro-[[Inflammation|inflammatory]] [[Cytokine|cytokines]] leading to a further exaggeration of [[atherosclerosis]] which is responsible for [[myocardial infarction]], [[Cerebrovascular accident|cerebrovascular]] accidents, etc in these patients. The increase in [[adhesion]] of [[granulocytes]] in patients with the JAK2V617F mutation on [[integrin]] @4B1, the [[ligand]] of VLA4 (either attached to a support or in a soluble form) has been shown in recent studies. In the mononuclear mutated cells, inhibition of activated Ras-proximate-1(small G- protein Rap 1) showed a reduction in [[cell adhesion]], thereby proving that the JAK2V167F mutation is the cause of increased [[integrin]] expression (@4B1). | |||
- NETs([[Neutrophil]] Extracellular Traps): Decondensed [[DNA]] along with [[Histone|histones]] are responsible for activation of [[Platelet|platelets]], inhibition of [[anticoagulation]] molecules, and activation of the intrinsic coagulation pathway by [[factor XII]] activation. | |||
The | *'''The role of red blood cells:''' | ||
- The Cytoreductive therapy in PV (CYTO-PV) clinical trial showed there is an increase in the risk of [[cardiovascular]] events in patients with >45% [[hematocrit]]; consequences depend on [[arterial]] or venous territory involvement. An amplified interaction between Lu/[[BCAM]] ([[erythroid]] Lutheran/ [[Basal cell]] [[adhesion]] molecule) and [[laminin]] accounts for qualitative abnormalities in red blood cells. | |||
The | *'''The role of endothelial cells:''' | ||
- Activation of [[endothelial cells]] leading to increased levels of [[thrombomodulin]], [[von Willebrand factor]], both E and P [[Selectin|selectins]], and circulating [[endothelial]] cells. Some studies have shown that the enzyme [[heparanase]] leads to [[tissue factor]] inhibitor dissociation leading to pro coagulation. Both [[heparanase]] and [[tissue factor]] inhibitor have been found in increasing quantities in [[bone marrow]] samples of patients suffering from PV. | |||
* | *'''The role of plasma:''' | ||
- [[D-dimer|D-dimers]], thrombin-anti-thrombin complexes, F1 and F2 [[fibrinogen]] fragments are found to be in increased quantities. A reduction in serum [[protein C]] and S along with increased resistance to activated [[protein C]] is also noted. Extracellular vesicles of cytoplasmic membrane remnants called microparticles isolated from patients with JAK2617F mutation have shown to increase [[thrombin]] production. <ref name="urlLa thrombose au cours des néoplasies myéloprolifératives - Influence de la mutation JAK2V617F | médecine/sciences">{{cite web |url=https://www.medecinesciences.org/en/articles/medsci/full_html/2019/08/msc190153/msc190153.html |title=La thrombose au cours des néoplasies myéloprolifératives - Influence de la mutation JAK2V617F | médecine/sciences |format= |work= |accessdate=}}</ref> | |||
==Genetics== | ==Genetics== | ||
*Majority of cases of [[polycythemia]] are inherited through [[somatic]] gene [[Mutation|mutations]]. Rarely, it can occur in [[Germ cell|germ cells]], in which case the inheritance is [[autosomal dominant]]. It must be noted that the most common mutations such as [[Janus kinase|JAK2]] or TET2 merely increase the risk of one developing the disease, not every person that has or inherits the [[mutation]] will develop it. <ref name="urlPrimary familial and congenital polycythemia | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program">{{cite web |url=https://rarediseases.info.nih.gov/diseases/9843/primary-familial-and-congenital-polycythemia |title=Primary familial and congenital polycythemia | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program |format= |work= |accessdate=}}</ref> | |||
The development of [[polycythemia]] is the result of multiple [[genetic mutations]] such as: | |||
*[ | |||
*[ | *[[JAK2]]V617F | ||
*MPL | |||
*CALR | |||
*[[CSF3R]] <ref name="pmid31778606">{{cite journal |vauthors=Jang MA, Choi CW |title=Recent insights regarding the molecular basis of myeloproliferative neoplasms |journal=Korean J Intern Med |volume=35 |issue=1 |pages=1–11 |date=January 2020 |pmid=31778606 |pmc=6960053 |doi=10.3904/kjim.2019.317 |url=}}</ref> | |||
==Associated Conditions== | |||
Conditions associated with [[polycythemia]] include: | |||
*[[Hereditary hemochromatosis]] <ref name="pmid31143309">{{cite journal |vauthors=Asif S, Begemann M, Raza S |title=Polycythemia in Patients With Hereditary Hemochromatosis: Real or Myth? |journal=J Clin Med Res |volume=11 |issue=6 |pages=422–427 |date=June 2019 |pmid=31143309 |pmc=6522237 |doi=10.14740/jocmr3816 |url=}}</ref> | |||
==Microscopic Pathology== | |||
*[ | *[[Peripheral blood smear]] findings are divided according to stages in the disease: | ||
Pre and overt [[polycythemia]]- [[normochromic]] and [[Normocytic anemia|normocytic]] red blood cells, [[hypochromic]] microcytic pattern if coexisting [[iron deficiency anemia]]. Elevation of [[Platelet|platelets]] and [[leukocytes]] especially [[Neutrophil|neutrophils]]. Post [[polycythemia]]- [[Teardrop cell|teardrop]] red blood cells, [[poikilocytosis]], [[nucleated]] [[Red blood cell|red blood cells]]. | |||
*[ | *[[Bone marrow]]- increased cellularity with panproliferation. | ||
== | Prepolycythemia- erythrocytosis | ||
Overt [[polycythemia]]- increased [[RBC]] mass | |||
Post [[polycythemia]] with [[fibrosis]]- increased [[reticulin]] deposition <ref name="pmid32491592">{{cite journal |vauthors=Lu X, Chang R |title= |journal= |volume= |issue= |pages= |date= |pmid=32491592 |doi= |url=}}</ref> | |||
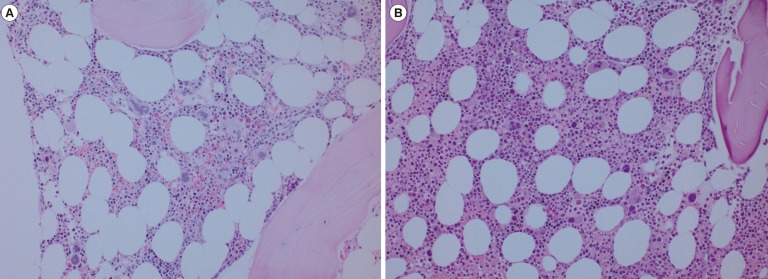
[[File:Polycythemia vera (PV).jpg|center|thumb|750x750px|'''Bone marrow histomorphology of essential thrombocythemia (ET) and masked polycythemia vera (PV). (A) Bone marrow morphology of ET shows normocellular marrow with an increased number of large and mature megakaryocytes. Note the hyperlobulated megakaryocytes without significant pleomorphism (bone marrow biopsy, hematoxylin, and eosin [H&E] stain, ×200). (B) Bone marrow morphology of masked PV (patient 1) shows a trilineage proliferation. Note the higher cellularity compared with that in (A) and an increased number of megakaryocytes displaying cytologic pleomorphism with mild atypia (bone marrow biopsy, H&E stain, ×200). Case courtesy by Daehyun Chu.''' {{Cite web}}|alt=]] | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
[[Category:Hematology]] | [[Category:Hematology]] | ||
[[Category:Emergency medicine]] | [[Category:Emergency medicine]] | ||
| Line 95: | Line 83: | ||
[[Category:Blood disorders]] | [[Category:Blood disorders]] | ||
[[Category:Up-To-Date]] | [[Category:Up-To-Date]] | ||
{{WS}} | {{WS}} | ||
{{WH}} | {{WH}} | ||
Latest revision as of 16:09, 26 February 2021
|
Polycythemia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Polycythemia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Polycythemia pathophysiology |
|
Risk calculators and risk factors for Polycythemia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Debduti Mukhopadhyay, M.B.B.S[2]
Overview
- The main mechanism is a valine to phenylalanine substitution, particularly the JAK2V617F mutation which leads to increased red blood cells. Other mechanisms include platelets, endothelial compartment, leukocytes, and plasma factors.
Pathophysiology
Physiology
- Polycythemia is considered a diagnosis when hematocrit is >48% in women and >52% in men, and when hemoglobin is >16.5g/dL in women and >18.5g/dL in men. [1]
- It is important that we know the similarities and differences between polycythemia and erythrocytosis.
- Similarities :
Both are characterized by an increase in red blood cells in the blood. Genetics may play a role in both disorders.
- Differences :
Polycythemia is the increase in red blood cells and hemoglobin above normal. Erythrocytosis is the increase in the mass of red blood cells. Polycythemia may show an increase in white blood cells and platelets as well. Increase in mass is limited to red blood cells only. [2]
Pathogenesis
- The main mechanism by which polycythemia vera develops is a valine to phenylalanine substitution, precisely the JAK2V617F leading to constitutive activation of cytokine receptors. This mutation is present in over 90% of patients with PV, 50% to 60% in patients with primary myelofibrosis, and 50% with patients with essential thrombocythemia.[3]
- Other factors contributing to the pathophysiology of polycythemia (amongst other myeloproliferative neoplasms) are blood cells, plasma factors, and the endothelial compartment.
- The role of platelets:
- Several studies in people and in mouse models have shown the increase in platelet activation and coagulation by factors such as cell surface proteins namely; P-selectin (CD62P), or tissue factor(CD142), and circulating leuco-platelets aggregates.
An increase in CD40 ligand, beta-thromboglobulin, platelet factor 4, thromboxane A2, and an increased expression of surface phosphatidylserine has been noted.
- The role of leukocytes:
- An increased expression of CD11, CD14, and leukocyte alkaline phosphatase, which is further amplified in the event of a JAK2V617F mutation. The mutated macrophages also produce pro-inflammatory cytokines leading to a further exaggeration of atherosclerosis which is responsible for myocardial infarction, cerebrovascular accidents, etc in these patients. The increase in adhesion of granulocytes in patients with the JAK2V617F mutation on integrin @4B1, the ligand of VLA4 (either attached to a support or in a soluble form) has been shown in recent studies. In the mononuclear mutated cells, inhibition of activated Ras-proximate-1(small G- protein Rap 1) showed a reduction in cell adhesion, thereby proving that the JAK2V167F mutation is the cause of increased integrin expression (@4B1). - NETs(Neutrophil Extracellular Traps): Decondensed DNA along with histones are responsible for activation of platelets, inhibition of anticoagulation molecules, and activation of the intrinsic coagulation pathway by factor XII activation.
- The role of red blood cells:
- The Cytoreductive therapy in PV (CYTO-PV) clinical trial showed there is an increase in the risk of cardiovascular events in patients with >45% hematocrit; consequences depend on arterial or venous territory involvement. An amplified interaction between Lu/BCAM (erythroid Lutheran/ Basal cell adhesion molecule) and laminin accounts for qualitative abnormalities in red blood cells.
- The role of endothelial cells:
- Activation of endothelial cells leading to increased levels of thrombomodulin, von Willebrand factor, both E and P selectins, and circulating endothelial cells. Some studies have shown that the enzyme heparanase leads to tissue factor inhibitor dissociation leading to pro coagulation. Both heparanase and tissue factor inhibitor have been found in increasing quantities in bone marrow samples of patients suffering from PV.
- The role of plasma:
- D-dimers, thrombin-anti-thrombin complexes, F1 and F2 fibrinogen fragments are found to be in increased quantities. A reduction in serum protein C and S along with increased resistance to activated protein C is also noted. Extracellular vesicles of cytoplasmic membrane remnants called microparticles isolated from patients with JAK2617F mutation have shown to increase thrombin production. [4]
Genetics
- Majority of cases of polycythemia are inherited through somatic gene mutations. Rarely, it can occur in germ cells, in which case the inheritance is autosomal dominant. It must be noted that the most common mutations such as JAK2 or TET2 merely increase the risk of one developing the disease, not every person that has or inherits the mutation will develop it. [5]
The development of polycythemia is the result of multiple genetic mutations such as:
Associated Conditions
Conditions associated with polycythemia include:
Microscopic Pathology
- Peripheral blood smear findings are divided according to stages in the disease:
Pre and overt polycythemia- normochromic and normocytic red blood cells, hypochromic microcytic pattern if coexisting iron deficiency anemia. Elevation of platelets and leukocytes especially neutrophils. Post polycythemia- teardrop red blood cells, poikilocytosis, nucleated red blood cells.
- Bone marrow- increased cellularity with panproliferation.
Prepolycythemia- erythrocytosis Overt polycythemia- increased RBC mass Post polycythemia with fibrosis- increased reticulin deposition [8]
References
- ↑ "Polycythemia Symptoms, Causes, Treatment & Diagnosis".
- ↑ "Difference Between Polycythemia and Erythrocytosis | Doctor HQ".
- ↑ "StatPearls". 2021. PMID 32491592 Check
|pmid=value (help). - ↑ "La thrombose au cours des néoplasies myéloprolifératives - Influence de la mutation JAK2V617F | médecine/sciences".
- ↑ "Primary familial and congenital polycythemia | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program".
- ↑ Jang MA, Choi CW (January 2020). "Recent insights regarding the molecular basis of myeloproliferative neoplasms". Korean J Intern Med. 35 (1): 1–11. doi:10.3904/kjim.2019.317. PMC 6960053 Check
|pmc=value (help). PMID 31778606. - ↑ Asif S, Begemann M, Raza S (June 2019). "Polycythemia in Patients With Hereditary Hemochromatosis: Real or Myth?". J Clin Med Res. 11 (6): 422–427. doi:10.14740/jocmr3816. PMC 6522237 Check
|pmc=value (help). PMID 31143309. - ↑ Lu X, Chang R. PMID 32491592 Check
|pmid=value (help). Missing or empty|title=(help)