Phosphorus oxoacids: Difference between revisions
| Line 145: | Line 145: | ||
=Metaphosphoric acid= | =Metaphosphoric acid= | ||
When an average of one molecule of water per phosphoric unit has been driven off, the resulting substance is a glassy solid having an empirical formula of '''HPO<sub>3</sub>''' and is called '''metaphosphoric acid'''.<ref>[http://www.bartleby.com/65/ph/phsphracid.html phosphoric acid. The Columbia Encyclopedia, Sixth Edition. 2001-05]</ref> Metaphosphoric acid is a singly anhydrous version of orthophosphoric acid and is sometimes used as a water- or moisture-absorbing reagent. Metaphosphoric acid has phosphorus in the formal oxidation state of +5 and is CID 3084658<ref name=metaphosphoric>{{ cite web |title=metaphosphoric acid - PubChem Public Chemical Database |url=http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3084658&loc=ec_rcs }}</ref>. | When an average of one molecule of water per phosphoric unit has been driven off, the resulting substance is a glassy solid having an empirical formula of '''HPO<sub>3</sub>''' and is called '''metaphosphoric acid'''.<ref>[http://www.bartleby.com/65/ph/phsphracid.html phosphoric acid. The Columbia Encyclopedia, Sixth Edition. 2001-05]</ref> Metaphosphoric acid is a singly anhydrous version of orthophosphoric acid and is sometimes used as a water- or moisture-absorbing reagent. Metaphosphoric acid has phosphorus in the formal oxidation state of +5 and is CID 3084658<ref name=metaphosphoric>{{ cite web |title=metaphosphoric acid - PubChem Public Chemical Database |url=http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=3084658&loc=ec_rcs }}</ref>. Although it has several CAS numbers: 10343-62-1, 135306-83-1, 13566-25-1, and 37267-86-0, only the last two are associated with the IUPAC name phosphenic acid. | ||
Phosphoric acid units can be bonded together in rings (cyclic structures), chains (catena), or branched by condensation yielding water as each acid is added. These structures consist of linked metaphosphoric acid molecules. For example, '''trimetaphosphoric acid''' on the page: [[Phosphoric acids and phosphates#Cyclo- or metaphosphoric acids and metaphosphates]] is the simplest cyclophosphoric acid. Metaphosphoric acid would need to share the doubly bonded oxygen with neighboring phosphorus atoms to form polymers itself. | |||
=Paraphosphoric acid= | =Paraphosphoric acid= | ||
Revision as of 08:03, 12 April 2009
Editor-In-Chief: Henry A. Hoff
Overview
Phosphorus forms a variety of compounds with oxygen and a number of these fit the description of an oxoacid. A phosphorus oxoacid contains phosphorus and oxygen, at least one hydrogen atom bound to oxygen, and forms an ion by the loss of one or more protons.
Phosphenous acid

Phosphenous acid (HOPO) is structurally based on σ2λ3 phosphorus.[1] CID 22497.[2] With only one hydroxyl radical per molecule, phosphenous acid can form the dimer, diphosphenous acid, with the release of one H2O. But, phosphenous acid does not yield H2O when it forms an oligomer with more molecules than diphosphenous acid. Instead the double bonded oxygen is shared with the adjacent phosphorus atom. In this way phosphenous acid can form polymers, including cyclic, cyclooligophosphenous acid, and branched, ultraoligophosphenous acid. An easily obtained cyclic trimer of phosphenous acid is composed of (HOPO)x repeat units where x=3, and OH is replaced by 2,6-di-tert-butyl-4-methylphenyl (Ar).[3]
Phosphinic acid
Phosphinic acid, HOP(O)H2, is a tautomer that exists in equilibrium with the minor tautomer hypophosphorous acid, HP(OH)2. Phosphinic acid is IUPAC name hydroxy(oxo)phosphanium, CAS Number 6303-21-5, CID 6326996, H2O2P+,[4] and CID 3085127, CAS Number 57583-56-9, H3O2P, with IUPAC name phosphinic acid. Both tautomers are described on the same page: Hypophosphorous acid. Usually, hypo and ous when added signify that the acid contains two less oxygen atoms than the "ic", phosphoric acid. Phosphinic acid is monoprotic as highlighted by its formula written as HOP(O)H2 and monobasic. Salts derived from these acids are called phosphinates or hypophosphites.
Hypophosphorous acid
The minor tautomer, HP(OH)2, does not have as yet a CID number, CAS number, or IUPAC name, but has been shown to be a stable tautomer.[5][6][7]
Hydroperoxyphosphane
Hydroperoxyphosphane (the IUPAC name) has the same formula, H3PO2, exists as H2POOH, CID 5260047, but has no CAS number.
Phosphorous acid
Phosphorous acid is sometimes called phosphorus acid, orthophosphorous acid, or monophosphorous acid. Phosphorous acid is a minor tautomer that exists in equilibrium with phosphonic acid. Both tautomers have their own pages: Phosphonic acid and Phosphorous acid, but because preparation and uses of 'phosphorous acid' actually pertain more to the major tautomer, phosphonic acid, and it is more often referred to as 'phosphorous acid', this added information can be found under phosphorous acid. Phosphorous acid has the chemical formula H3PO3, which is best expressed as P(OH)3 to show its triprotic character. P(OH)3 (IUPAC: phosphorous acid) has CAS number 10294-56-1 and CID: 107909[8]. It has been shown to be a stable tautomer.[9][5]
Diphosphorous acid is made by condensation:
2P(OH)3 <=> (OH)2POP(OH)2 + H20
For phosphorous acid, an oligophosphorous acid refers to a few molecules condensed into a molecule with the loss of H2O as each unit of P(OH)3 is added on. The general formula is
(HO)2PO[P(OH)O]n-2P(OH)2,
where n = 2, 3, 4, etc., oligo-. Here as with phosphonic acid the repeat unit is (HPO2)n-2.
A polyphosphorous acid can have dozens of units condensed in a row. Regardless of the value of n, both polyphosphorous acid and polyphosphonic acid have the same chemical formula for any specific n, e.g., triphosphosphonic acid is H5P3O7 and triphosphorous acid is H5P3O7 for n=3.
In oligophosphorous acids of sufficient size, there are multiple OH that can result in the condensation of a cyclophosphorous acid that does not have multiple (HPO3) metaphosphoric acid units.
However, the usual referral to a cyclophosphorous acid (cyclophosphites) may be misnomers wherein the cyclic portion is carbon-based with a phosphorous acid side chain of one or more molecules, or one or a limited number of either of the two tautomers included in the ring but as a minority contributor.
For example the effect of varying ring size on the phosphonate-phosphite tautomerism of cyclophosphorous acids has been shown.[10] But the cyclophosphorous acids are biheteroorganic.
Branching can also occur in either oligophosphorous or polyphosphorous acid. These are ultraoligophosphorous or ultrapolyphosphorous acids, or ultraoligophosphites and ultrapolyphosphites, respectively.
Phosphoric acid
Phosphoric acid, orthophosphoric acid, or monophosphoric acid has its own page: Phosphoric acid. Other acids commonly called 'phosphoric acid' are on page: Phosphoric acids and phosphates. Two of the oligophosphoric acids also have their own pages: diphosphoric acid or Pyrophosphoric acid and Triphosphoric acid or tripolyphosphoric acid. Generally, oligophosphoric and polyphosphoric acids are discussed on the page: Phosphoric acids and phosphates. Phosphoric acids can occur in cyclic form, cyclooligophosphoric acids, branched, ultraphosphoric acids, or combinations of both, usually referred to as polyphosphoric acids. See the page: Phosphoric acids and phosphates for more information, especially phosphoric anhydride.
Metaphosphoric acid
When an average of one molecule of water per phosphoric unit has been driven off, the resulting substance is a glassy solid having an empirical formula of HPO3 and is called metaphosphoric acid.[11] Metaphosphoric acid is a singly anhydrous version of orthophosphoric acid and is sometimes used as a water- or moisture-absorbing reagent. Metaphosphoric acid has phosphorus in the formal oxidation state of +5 and is CID 3084658[12]. Although it has several CAS numbers: 10343-62-1, 135306-83-1, 13566-25-1, and 37267-86-0, only the last two are associated with the IUPAC name phosphenic acid.
Phosphoric acid units can be bonded together in rings (cyclic structures), chains (catena), or branched by condensation yielding water as each acid is added. These structures consist of linked metaphosphoric acid molecules. For example, trimetaphosphoric acid on the page: Phosphoric acids and phosphates#Cyclo- or metaphosphoric acids and metaphosphates is the simplest cyclophosphoric acid. Metaphosphoric acid would need to share the doubly bonded oxygen with neighboring phosphorus atoms to form polymers itself.
Paraphosphoric acid
Paraphosphoric acid (H5PO5) is obtained by burning phosphorus in dry air, or oxygen gas, appears in small crystals, like snow, and is formed, combined with water, by heating to redness pyrophosphoric and phosphoric acids; when fused, it cools into a brittle and transparent solid, resembling ice.[13] Paraphosphoric acid has phosphorus in the formal oxidation state of +5.
Thiophosphoric acid
Thiophosphoric acids have one or more oxygens replaced by sulfur. An example of a thiophosphoric acid ester (thiophosphorate) is on the page: Diazinon. Monothiophosphoric acid has the molecular formula H3PO3S, P(O)(OH)2(SH), is SID 50009326 and CID 167254 114 Da.[14] Dithiophosphoric acid has the molecular formula H3PO2S2, P(S)(OH)2(SH), is CAS number 15834-33-0, SID 729652 and CID 152119 130 Da.[15] A trithiophosphoric acid is phosphorotrithioic acid with the molecular formula H9C3OPS3, actually the methyl ester S,S,S,-trimethyl phophorotrithioate, H3CSP(=O)(SCH3)SCH3, CID 120321, IUPAC Name: bis(methylsulfanyl)phosphorylsulfanylmethane, 188 Da, CAS number 681-71-0.[16]
Perphosphoric acid
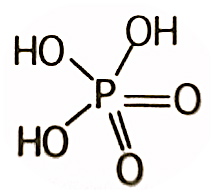
The use of per before the phosphoric indicates that the acid contains one more oxygen than phosphoric acid. For example, permonophosphoric acid has the formula H3PO5 instead of H3PO4 for orthophosphoric acid (monophosphoric acid).
Permonophosphoric acid is an acid formed when pyrophosphoric acid is treated with a large excess of hydrogen peroxide.
H4P2O7 + 2H2O2 <=> 2H3PO5 + H2O
Permonophosphoric acid 114 Da is a triprotic acid, yielding PO53- (permonophosphate ion) with its H+s removed. The preparation of permonophosphoric acid, H3PO5, was obtained by J. Schmidlin and P. Massini (Ber., 1910, 43, 1162).[17] Monoperphosphoric acid can be obtained when phosphorus pentoxide is treated with 30 % hydrogen peroxide, while cooled with ice-water.[18] But there are other forms of H3PO5.
When two oxygens are linked as in hydrogen peroxide, the permonophosphoric acid formed is peroxomonophosphoric acid (PMPA), (OH)2O=P-O-O-H, H3PO5. PMPA can be prepared by the perchloric acid hydrolysis of potassium peroxodiphosphate.[19] The species H3PO5, H2PO5-, HPO52-, and PO53- become predominant in this order with increasing pH and the electrophilic character of these species decreases.[19] PMPA exists as H2PO5- and HPO52- in the pH range 4-7.[19]
Monoperphosphoric acid and permonophosphoric acid are the same acid. Should the phosphoric acid be either an oligophosphoric acid or polyphosphoric acid as discussed below, the location of per may indicate different acids. For instance, perdiphosphoric acid would be H4P2O8, one more oxygen added to pyrophosphoric acid. But diperphosphoric acid could refer to two permonophosphoric acid molecules condensed with the loss of H2O to yield H4P2O9.
Superphosphoric acid
Superphosphoric acid is a blend of orthophosphoric and polyphosphoric acid, CAS Number 8017-16-1. Superphosphoric acids can be in the concentration range of 62-85% P2O5 (86-117% H3PO4), but at about 90% H3PO4 the amount of condensed phosphate species becomes significant.[20] The concentration range of the superphosphoric acids is a range of increasing importance in fertilizer technology.[20]
Superphosphoric acid is sometimes called multiphosphoric acid or polyphosphoric acid.
Hyperphosphoric acid
Hyperphosphoric acid is concentrated phosphoric acid. It can be prepared in two ways by:
(1) producing phosphorus pentoxide electrothermically and dissolving this oxide in a small quantity of water or
(2) concentrating phosphoric acid obtained by wet treatment with hot gas at temperatures above 700°C.[21]
It has also been called oleophosphoric acid.[21]
The first process produces hyperphosphoric acid of high purity and concentration but is very expensive. The second process produces a cheaper acid but introduces several impurities, among which are fluorine and sulphuric acid, and various metals, e.g. aluminium and iron. The metallic impurities exist as complexes in the form of polyphosphoric salts.[21]
Hyperphosphoric acid can be added to blood to release CO2.[22]
References
- ↑ Ronald Stanley Edmundson (1988). Dictionary of Organophosphorus Compounds. CRC Press. p. 1347. ISBN 0412257904, 9780412257902 Check
|isbn=value: invalid character (help). Text "page xi " ignored (help) - ↑ "Phosphenous acid - PubChem Public Chemical Database".
- ↑ Quin LD, Jankowski S, Rudzinski J, Sommese AG, Wu XP (1993). "Experiments on the generation of 2-coordinate phosphoryl species by fragmentation of 7-phosphanorbornene and 3-phospholene derivatives". J Org Chem. 58 (23): 6212–6. doi:10.1021/jo00075a014. Unknown parameter
|month=ignored (help) - ↑ "Phosphinic acid - PubChem Public Chemical Database".
- ↑ 5.0 5.1 Sokolov MN, Chubarova EV, Kovalenko KA, Mironov IV, Virovets1 AV, Peresypkina EV, Fedin VP (2005). "Stabilization of tautomeric forms P(OH)3 and HP(OH)2 and their derivatives by coordination to palladium and nickel atoms in heterometallic clusters with the Mo3MQ44+ core (M = Ni, Pd; Q = S, Se)". Russian Chem Bull. 54: 615. doi:10.1007/s11172-005-0296-1.
- ↑ Nonoyama M (2003). "Separating Hypophosphorous Acid HP(OH)2, Phosphorous Acid P(OH)3, and Arsenous Acid As(OH)3 as Complexes". Kagaku to Kogyo. 56 (9): 998.
- ↑ Akbayeva DN, Di Vaira M, Costantini SS, Peruzzini M, Stoppioni P (2006). "Stabilization of the tautomers HP(OH)2 and P(OH)3 of hypophosphorous and phosphorous acids as ligands". Dalton Trans. (2): 389–95. PMID 16365654. Unknown parameter
|month=ignored (help) - ↑ "Phosphorous acid - PubChem Public Chemical Database".
- ↑ Xi C, Liu Y, Lai C, Zhou L (2004). "Synthesis of molybdenum complex with novel P(OH)3 ligand based on the one-pot reaction of Mo(CO)6 with HP(O)(OEt)2 and water". Inorgn Chem Commun. 7 (11): 1202–4. doi:10.1016/j.inoche.2004.09.012.
- ↑ Gladyshev EN, Bayushkin PY, Sokolov VS (1978). "Some one-electron oxidation reactions of biheteroorganic derivatives with Ge-Hg and Ge-Li groupings". Russ Chem Bull. 27 (3): 592–5. doi:10.1007/BF00923949. Unknown parameter
|month=ignored (help) - ↑ phosphoric acid. The Columbia Encyclopedia, Sixth Edition. 2001-05
- ↑ "metaphosphoric acid - PubChem Public Chemical Database".
- ↑ Alonzo Gray (2008). Elements of Chemistry. BiblioBazaar, LLC. p. 205. ISBN 0559049102, 9780559049101 Check
|isbn=value: invalid character (help). More than one of|pages=and|page=specified (help) - ↑ "thiophosphoric acid - Substance Summary - PubChem Public Chemical Database".
- ↑ "phosphorodithioic acid - Compound Summary - PubChem Public Chemical Database".
- ↑ "S,S,S-trimethyl phosphorotrithioate - Compound Summary - PubChem Public Chemical Database".
- ↑ Ogata Y, Urasaki I, Nagura K, Satomi N (1974). "Kinetics of the aromatic hydroxylation with permonophosphoric acid". Tetrahedron. 30 (17): 3021–5. doi:10.1016/S0040-4020(01)97547-7 .
- ↑ Joseph William Mellor (1912). Modern Inorganic Chemistry. Longmans, Green. p. 597. More than one of
|pages=and|page=specified (help) - ↑ 19.0 19.1 19.2 Panigrahi GP, Panda R (1980). "Oxidation studies with peroxomonophosphoric acid. III. A kinetic and mechanistic study of oxidation of dialkyl sulfides". Bull Chem Soc Jpn. 53 (8): 2366–70.
- ↑ 20.0 20.1 Luff BB, Reed RB, Wakefield ZT (1971). "Enthalpy of dilution of superphosphoric acids". J Chem Eng Data. 16 (3): 342–4. doi:10.1021/je60050a033. Unknown parameter
|month=ignored (help) - ↑ 21.0 21.1 21.2 "Process for obtaining concentrated phosphoric acid".
- ↑ Sidossis LS, Mittendorfer B, Chinkes D, Walser E, Wolfe RR (1999). "Effect of hyperglycemia-hyperinsulinemia on whole body and regional fatty acid metabolism". Am J Physiol. 276 ((3 Pt 1)): E427–34. PMID 10070006. Unknown parameter
|month=ignored (help)