Phosphonic acid
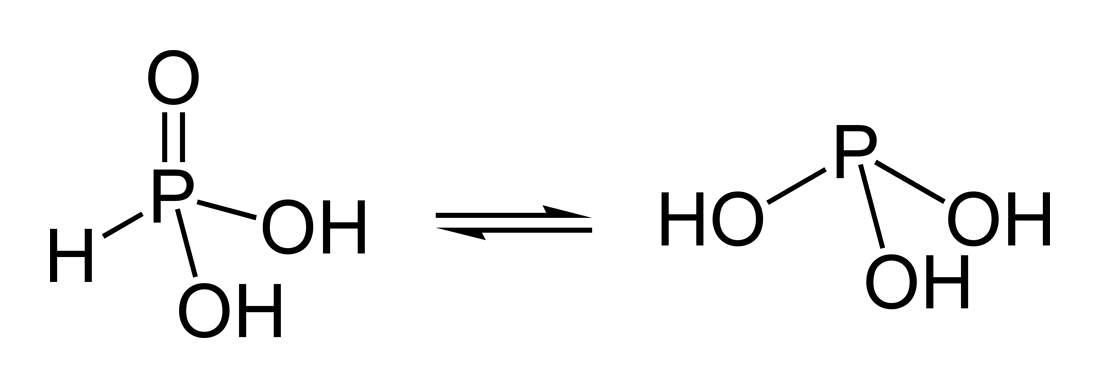
phosphonic acid (left)
phosphorous acid (right)
In inorganic chemistry, phosphonic acid is a phosphorus oxoacid with a formula of H3PO3, more commonly known as phosphorous acid. It exists in solution as two tautomers, the major one being HP(O)(OH)2 and the minor one P(OH)3. The former is sometimes termed phosphonic acid, with the latter designated as phosphorous acid. Sometimes confusingly, both these names are also used to refer to H3PO3 in general, i.e. both tautomers.
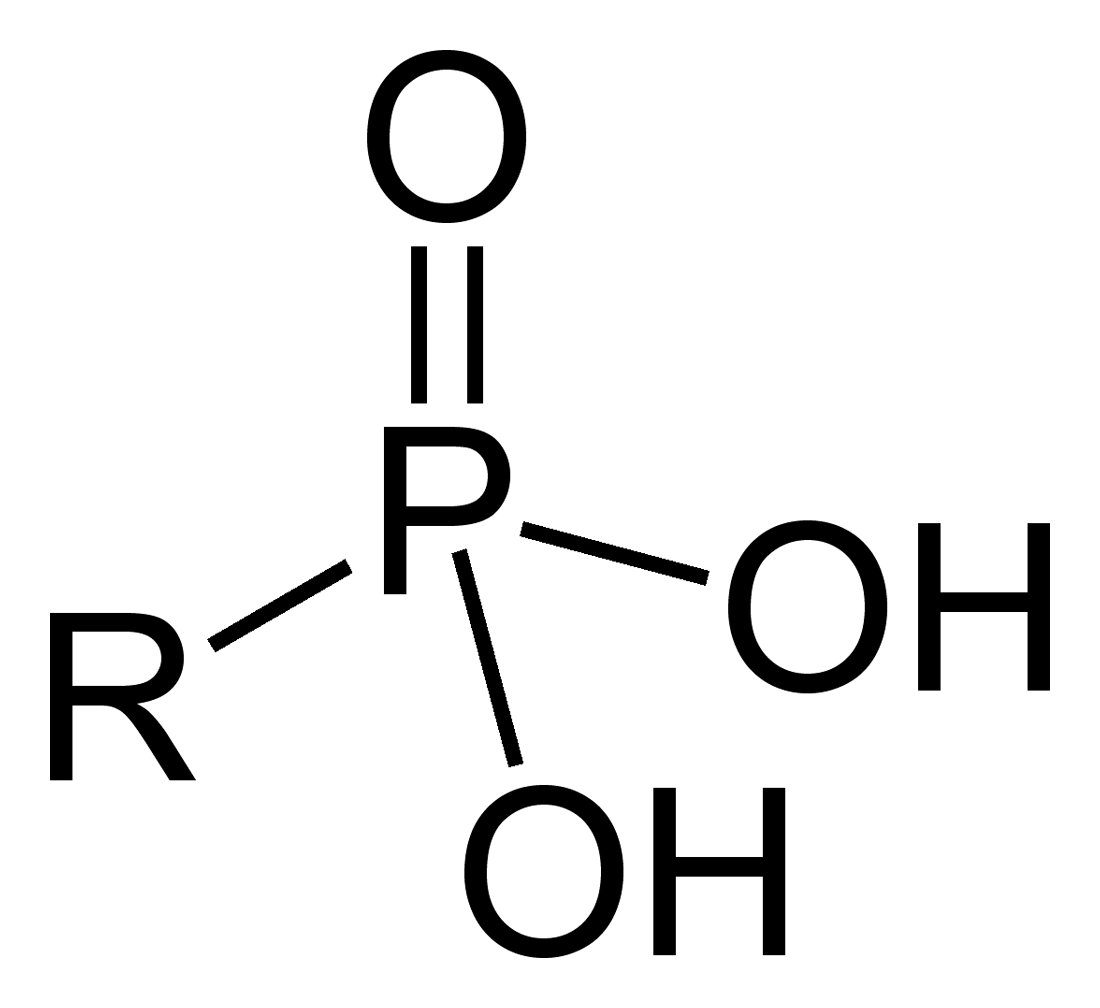
In organic chemistry, a phosphonic acid is a compound with the general formula RP(O)(OH)2.
An example of an organic phosphonic acid is Foscarnet.
An oligophosphonic acid refers to a few molecules of phosphonic acid condensed into a molecule with the loss of water; for example, ethane-1,1,2-triphosphonic acid[1]. In some phosphonic anhydrides (RPO2)3, R can be tBu, 2-methylphenyl, 2,4,6-trimethylphenyl.[2]
References
- ↑ Bogdán Cs, Péczely G, Hägele G (2007). "Metal Complexes of Ethane and Propane Frame-Substituted Oligophosphonic and Oligophosphonocarboxylic Acids". Phosphorus, Sulfur, and Silicon and the Related Elements. 182 (10): 2337–50. Unknown parameter
|month=ignored (help) - ↑ Diemert K, Kuchen W, Poll W, Sandt F (1998). "A Convenient Synthesis of Phosphonic Anhydrides - Trimers [RPO2]3 (R = tert-Butyl, 2-Methylphenyl, 2,4,6-Trimethylphenyl): Their Structures and Reaction Products". Eur J Inorgan Chem. 1998 (3): 361–6. doi:10.1002/(SICI)1099-0682(199803)1998:3<361::AID-EJIC361>3.0.CO;2-T .
See also
External links
- Phosphonic+Acid at the US National Library of Medicine Medical Subject Headings (MeSH)
- PubChem