Pentosan polysulfate: Difference between revisions
m (Protected "Pentosan polysulfate": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
|IUPAC_name = | |authorTag= | ||
| image = Pentosan polysulfate. | |||
{{VP}} | |||
<!--Overview--> | |||
|genericName= | |||
Pentosan polysulfate | |||
|aOrAn= | |||
a | |||
|drugClass= | |||
[[cystitis]] agent | |||
|indication= | |||
[[interstitial cystitis]], chronic, relief of [[bladder pain]] or discomfort | |||
|hasBlackBoxWarning= | |||
|adverseReactions= | |||
[[alopecia]], [[rash]], [[abdominal pain]], [[diarrhea]], [[indigestion]], [[nausea]], increased [[liver enzymes]], [[dizziness]], [[headache]] | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Interstitial cystitis, chronic, relief of bladder pain or discomfort===== | |||
* Dosing Information | |||
:*ELMIRON® (pentosan polysulfate sodium) is indicated for the relief of bladder pain or discomfort associated with [[interstitial cystitis]]. | |||
:*The recommended dose of ELMIRON® is 300 mg/day taken as one 100 mg capsule orally three times daily. The capsules should be taken with water at least 1 hour before meals or 2 hours after meals. | |||
:*Patients receiving ELMIRON® should be reassessed after 3 months. If improvement has not occurred and if limiting adverse events are not present, ELMIRON® may be continued for another 3 months. | |||
:*The clinical value and risks of continued treatment in patients whose pain has not improved by 6 months is not known. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
* ELMIRON® is contraindicated in patients with known [[hypersensitivity]] to the drug, structurally related compounds, or excipients. | |||
<!--Warnings--> | |||
|warnings= | |||
*None. | |||
====Precautions==== | |||
* General | |||
:*ELMIRON® is a weak anticoagulant (1/15 the activity of [[heparin]]). At a daily dose of 300 mg (n = 128), rectal hemorrhage was reported as an adverse event in 6.3% of patients. Bleeding complications of [[ecchymosis]], [[epistaxis]], and [[gum hemorrhage]] have been reported. Patients undergoing invasive procedures or having signs/symptoms of underlying [[coagulopathy]] or other increased risk of bleeding (due to other therapies such as [[coumarin]] anticoagulants, [[heparin]], [[t-PA]], [[streptokinase]], high dose [[aspirin]], or [[nonsteroidal anti-inflammatory drugs]]) should be evaluated for [[hemorrhage]]. Patients with diseases such as aneurysms, [[thrombocytopenia]], [[hemophilia]], [[gastrointestinal ulcerations]], [[polyps]], or [[diverticula]] should be carefully evaluated before starting ELMIRON®. | |||
:*A similar product that was given [[subcutaneously]], [[sublingually]], or [[intramuscularly]] (and not initially metabolized by the liver) is associated with delayed immunoallergic [[thrombocytopenia]] with symptoms of [[thrombosis]] and [[hemorrhage]]. Caution should be exercised when using ELMIRON® in patients who have a history of [[heparin induced thrombocytopenia]]. | |||
:*[[Alopecia]] is associated with pentosan polysulfate and with [[heparin]] products. In clinical trials of ELMIRON®, [[alopecia]] began within the first 4 weeks of treatment. Ninety-seven percent (97%) of the cases of [[alopecia]] reported were [[alopecia areata]], limited to a single area on the scalp. | |||
*Hepatic Insufficiency | |||
:*ELMIRON® has not been studied in patients with [[hepatic insufficiency]]. Because there is evidence of hepatic contribution to the elimination of ELMIRON®, [[hepatic impairment]] may have an impact on the pharmacokinetics of ELMIRON®. Caution should be exercised when using ELMIRON® in this patient population. | |||
:*Mildly (< 2.5 × normal) elevated [[transaminase]], [[alkaline phosphatase]], [[γ-glutamyl transpeptidase]], and [[lactic dehydrogenase]] occurred in 1.2% of patients. The increases usually appeared 3 to 12 months after the start of ELMIRON® therapy, and were not associated with [[jaundice]] or other clinical signs or symptoms. These abnormalities are usually transient, may remain essentially unchanged, or may rarely progress with continued use. Increases in [[PTT]] and [[PT]] (< 1% for both) or [[thrombocytopenia]] (0.2%) were noted. | |||
*Laboratory Test Findings | |||
:*Pentosan polysulfate sodium did not affect [[prothrombin time]] (PT) or [[partial thromboplastin time]] ([[PTT]]) up to 1200 mg per day in 24 healthy male subjects treated for 8 days. Pentosan polysulfate sodium also inhibits the generation of [[factor Xa]] in plasma and inhibits [[thrombin]]-induced [[platelet aggregation]] in human [[platelet]] rich plasma ex vivo. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*ELMIRON® was evaluated in clinical trials in a total of 2627 patients (2343 women, 262 men, 22 unknown) with a mean age of 47 [range 18 to 88 with 581 (22%) over 60 years of age]. Of the 2627 patients, 128 patients were in a 3–month trial and the remaining 2499 patients were in a long-term, unblinded trial. | |||
*Deaths occurred in 6/2627 (0.2%) patients who received the drug over a period of 3 to 75 months. The deaths appear to be related to other concurrent illnesses or procedures, except in one patient for whom the cause was not known. | |||
*Serious adverse events occurred in 33/2627 (1.3%) patients. Two patients had severe [[abdominal pain]] or [[diarrhea]] and [[dehydration]] that required hospitalization. Because there was not a control group of patients with [[interstitial cystitis]] who were concurrently evaluated, it is difficult to determine which events are associated with ELMIRON® and which events are associated with concurrent illness, medicine, or other factors. | |||
: [[File:{{PAGENAME}}03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*The adverse events described below were reported in an unblinded clinical trial of 2499 [[interstitial cystitis]] patients treated with ELMIRON®. Of the original 2499 patients, 1192 (48%) received ELMIRON® for 3 months; 892 (36%) received ELMIRON for 6 months; and 598 (24%) received ELMIRON® for one year, 355 (14%) received ELMIRON® for 2 years, and 145 (6%) for 4 years. | |||
*Frequency (1 to 4%): [[Alopecia]] (4%), [[diarrhea]] (4%), [[nausea]] (4%), [[headache]] (3%), [[rash]] (3%), [[dyspepsia]] (2%), [[abdominal pain]] (2%), liver function abnormalities (1%), [[dizziness]] (1%). | |||
*Frequency (≤ 1%): | |||
:*Digestive: [[Vomiting]], [[mouth ulcer]], [[colitis]], [[esophagitis]], [[gastritis]], [[flatulence]], [[constipation]], [[anorexia]], gum hemorrhage. | |||
:*Hematologic: [[Anemia]], [[ecchymosis]], increased [[prothrombin time]], increased [[partial thromboplastin time]], [[leukopenia]], [[thrombocytopenia]]. | |||
:*Hypersensitive Reactions: Allergic reaction, [[photosensitivity]]. | |||
:*Respiratory System: [[Pharyngitis]], [[rhinitis]], epistaxis, [[dyspnea]]. | |||
:*Skin and Appendages: [[Pruritus]], [[urticaria]]. | |||
:*Special Senses: [[Conjunctivitis]], [[tinnitus]], [[optic neuritis]], [[amblyopia]], [[retinal hemorrhage]]. | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
*[[Rectal Hemorrhage]] | |||
:*ELMIRON® was evaluated in a randomized, double-blind, parallel group, Phase 4 study conducted in 380 patients with [[interstitial cystitis]] dosed for 32 weeks. At a daily dose of 300 mg (n = 128), rectal hemorrhage was reported as an adverse event in 6.3% of patients. The severity of the events was described as "mild" in most patients. Patients in that study who were administered ELMIRON® 900 mg daily, a dose higher than the approved dose, experienced a higher incidence of [[rectal hemorrhage]], 15%. | |||
*Liver Function Abnormality | |||
:*A randomized, double-blind, parallel group, phase 2 study was conducted in 100 men (51 ELMIRON® and 49 placebo) dosed for 16 weeks. At a daily dose of 900 mg, a dose higher than the approved dose, elevated [[liver function tests]] were reported as an adverse event in 11.8% (n = 6) of ELMIRON®-treated patients and 2% (n = 1) of placebo-treated patients. | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
* In a study in which healthy subjects received pentosan polysulfate sodium 100 mg capsule or placebo every 8 hours for 7 days, and were titrated with warfarin to an INR of 1.4 to 1.8, the pharmacokinetic parameters of R-warfarin and S-warfarin were similar in the absence and presence of pentosan polysulfate sodium. INR for warfarin + placebo and warfarin + pentosan polysulfate sodium were comparable. | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category B''' | |||
*Reproduction studies have been performed in mice and rats with intravenous daily doses of 15 mg/kg, and in rabbits with 7.5 mg/kg. These doses are 0.42 and 0.14 times the daily oral human doses of ELMIRON® when normalized to body surface area. These studies did not reveal evidence of impaired fertility or harm to the fetus from ELMIRON®. Direct in vitro bathing of cultured mouse embryos with pentosan polysulfate sodium (PPS) at a concentration of 1 mg/mL may cause reversible limb bud abnormalities. Adequate and well-controlled studies have not been performed in pregnant women. Because animal studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed. | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
*It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ELMIRON® is administered to a nursing woman. | |||
|useInPed= | |||
*Safety and effectiveness in pediatric patients below the age of 16 years have not been established. | |||
|useInGeri= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Oral | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | |||
* Overdose has not been reported. Based upon the pharmacodynamics of the drug, toxicity is likely to be reflected as [[anticoagulation]], [[bleeding]], [[thrombocytopenia]], liver function abnormalities, and [[gastric distress]]. At a daily dose of 900 mg for 32 weeks (n = 127) in a clinical trial, [[rectal hemorrhage]] was reported as an adverse event in 15% of patients. At a daily dose of ELMIRON® 900 mg for 16 weeks in a clinical trial that enrolled 51 patients in the ELMIRON® group and 49 in the placebo group, elevated liver function tests were reported as an adverse event in 11.8% of patients in the ELMIRON® group and 2% of patients in the placebo group. | |||
====Management==== | |||
* In the event of acute overdosage, the patient should be given [[gastric lavage]] if possible, carefully observed and given symptomatic and supportive treatment. | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
{{Drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 464198430 | |||
| IUPAC_name = | |||
| image = Pentosan polysulfate.png | |||
| width = 150px | | width = 150px | ||
| | <!--Clinical data--> | ||
| | | tradename = | ||
| | | Drugs.com = {{drugs.com|CDI|pentosan_polysulfate}} | ||
| | | pregnancy_category = B | ||
| | | legal_status = | ||
| routes_of_administration = [[mouth|Oral]], [[intramuscular injection|intramuscular]], [[intra-articular]], [[Ventricle (heart)|intraventricular]] | |||
| bioavailability= | |||
| metabolism = | <!--Pharmacokinetic data--> | ||
| elimination_half-life= | | bioavailability = | ||
| excretion = | | metabolism = | ||
| | | elimination_half-life = | ||
| | | excretion = Urine | ||
| | |||
<!--Identifiers--> | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = NA | |||
| ChEMBL = 1201516 | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 37300-21-3 | |||
| CAS_supplemental = (free acid)<br />{{CAS|116001-96-8}} (sodium salt) | |||
| ATC_prefix = C05 | |||
| ATC_suffix = BA04 | |||
| ATC_supplemental = {{ATC|G04|BX15}} {{ATCvet|M01|AX90}} | |||
| PubChem = 37720 | |||
<!--Chemical data--> | |||
| chemical_formula = (C<sub>5</sub>H<sub>6</sub>Na<sub>2</sub>O<sub>10</sub>S<sub>2</sub>)<sub>n</sub> | |||
| molecular_weight = | |||
}} | }} | ||
<!--Mechanism of Action--> | |||
= | |mechAction= | ||
* Pentosan polysulfate sodium is a low molecular weight heparin-like compound. It has anticoagulant and fibrinolytic effects. The mechanism of action of pentosan polysulfate sodium in interstitial cystitis is not known. | |||
<!--Structure--> | |||
|structure= | |||
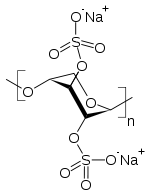
* Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative, which chemically and structurally resembles glycosaminoglycans. It is a white odorless powder, slightly hygroscopic and soluble in water to 50% at pH 6. It has a molecular weight of 4000 to 6000 Dalton with the following structural formula: | |||
: [[File:{{PAGENAME}}06.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*ELMIRON® is supplied in white opaque hard gelatin capsules containing 100 mg pentosan polysulfate sodium, microcrystalline cellulose, and magnesium stearate. It also contains pharmaceutical glaze (modified) in SD-45, synthetic black iron oxide, FD&C Blue No. 2 aluminum lake, FD&C Red No. 40 aluminum lake, FD&C Blue No. 1 aluminum lake, D&C Yellow No. 10 aluminum lake, n-butyl alcohol, propylene glycol, SDA-3A alcohol, and titanium dioxide. It is formulated for oral use. | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
*The mechanism by which pentosan polysulfate sodium achieves its effects in patients is unknown. In preliminary clinical models, pentosan polysulfate sodium adhered to the bladder wall mucosal membrane. The drug may act as a buffer to control cell permeability preventing irritating solutes in the urine from reaching the cells. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
*Absorption | |||
* | :*In a clinical pharmacology study in which healthy female volunteers received a single oral 300 or 450 mg dose of pentosan polysulfate sodium containing radiolabeled drug as a solution under fasted conditions, maximal levels of plasma radioactivity were seen approximately at a median of 2 hours (range 0.6-120 hours) after dosing. Based on urinary excretion of radioactivity, a mean of approximately 6% of a radiolabeled oral dose of pentosan polysulfate sodium is absorbed and reaches the systemic circulation. | ||
{{ | *Food Effects | ||
{{ | :*In clinical trials, ELMIRON® was administered with water 1 hour before or 2 hours after meals; the effect of food on absorption of pentosan polysulfate sodium is not known. | ||
*Distribution | |||
:*Preclinical studies with parenterally administered radiolabeled pentosan polysulfate sodium showed distribution to the uroepithelium of the genitourinary tract with lesser amounts found in the liver, spleen, lung, skin, periosteum, and bone marrow. Erythrocyte penetration is low in animals. | |||
*Metabolism | |||
:*The fraction of pentosan polysulfate sodium that is absorbed is metabolized by partial desulfation in the liver and spleen, and by partial depolymerization in the kidney to a large number of metabolites. Both the desulfation and depolymerization can be saturated with continued dosing. | |||
*Excretion | |||
:*Following administration of an oral solution of a 300 or 450 mg dose of pentosan polysulfate sodium containing radiolabeled drug to groups of healthy subjects, plasma radioactivity declined with mean half-lives of 27 and 20 hours, respectively. A large proportion of the orally administered dose of pentosan polysulfate sodium (mean 84% in the 300 mg group and 58% in the 450 mg group) is excreted in feces as unchanged drug. A mean of 6% of an oral dose is excreted in the urine, mostly as desulfated and depolymerized metabolites. Only a small fraction of the administered dose (mean 0.14%) is recovered as intact drug in urine. | |||
*Special Populations | |||
:*The pharmacokinetics of pentosan polysulfate sodium has not been studied in geriatric patients or in patients with hepatic or renal impairment. | |||
*Drug-Drug Interactions | |||
:*In a study in which healthy subjects received pentosan polysulfate sodium 100 mg capsule or placebo every 8 hours for 7 days, and were titrated with warfarin to an INR of 1.4 to 1.8, the pharmacokinetic parameters of R-[[warfarin]] and S-warfarin were similar in the absence and presence of pentosan polysulfate sodium. [[INR]] for [[warfarin]] + placebo and [[warfarin]] + pentosan polysulfate sodium were comparable. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
=====Carcinogenicity, Mutagenesis, Impairment of Fertility===== | |||
*Long term carcinogenicity studies of ELMIRON® in F344/N rats and B6C3F1 mice have been conducted. In these studies, ELMIRON® was orally administered once daily via gavage, 5 days per week, for up to 2 years. The dosages administered to mice were 56, 168 or 504 mg/kg. The dosages administered to rats were 14, 42, or 126 mg/kg for males, and 28, 84, or 252 mg/kg for females. The dosages tested were up to 60 times the maximum recommended human dose (MRHD) in rats, and up to 117 times the MRHD in mice, on a mg/kg basis. The results of these studies in rodents showed no clear evidence of drug-related tumorigenesis or carcinogenic risk. | |||
*Pentosan polysulfate sodium was not clastogenic or mutagenic when tested in the mouse micronucleus test or the Ames test (S. typhimurium). The effect of pentosan polysulfate sodium on spermatogenesis has not been investigated. | |||
<!--Clinical Studies--> | |||
|clinicalStudies= | |||
*ELMIRON® was evaluated in two clinical trials for the relief of pain in patients with chronic [[interstitial cystitis]] (IC). All patients met the NIH definition of IC based upon the results of [[cystoscopy]], cytology, and biopsy. One blinded, randomized, placebo-controlled study evaluated 151 patients (145 women, 5 men, 1 unknown) with a mean age of 44 years (range 18 to 81). Approximately equal numbers of patients received either placebo or ELMIRON® 100 mg three times a day for 3 months. Clinical improvement in bladder pain was based upon the patient's own assessment. In this study, 28/74 (38%) of patients who received ELMIRON® and 13/74 (18%) of patients who received placebo showed greater than 50% improvement in bladder pain (p = 0.005). | |||
*A second clinical trial, the physician's usage study, was a prospectively designed retrospective analysis of 2499 patients who received ELMIRON® 300 mg a day without blinding. Of the 2499 patients, 2220 were women, 254 were men, and 25 were of unknown sex. The patients had a mean age of 47 years and 23% were over 60 years of age. By 3 months, 1307 (52%) of the patients had dropped out or were ineligible for analysis, overall, 1192 (48%) received ELMIRON® for 3 months; 892 (36%) received ELMIRON® for 6 months; and 598 (24%) received ELMIRON® for one year. | |||
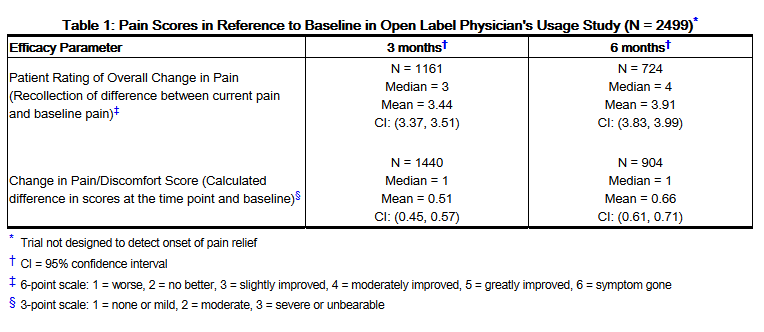
*Patients had unblinded evaluations every 3 months for the patient's rating of overall change in pain in comparison to baseline and for the difference calculated in "pain/discomfort" scores. At baseline, pain/discomfort scores for the original 2499 patients were severe or unbearable in 60%, moderate in 33% and mild or none in 7% of patients. The extent of the patients' pain improvement is shown in Table 1. | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*At 3 months, 722/2499 (29%) of the patients originally in the study had pain scores that improved by one or two categories. By 6 months, in the 892 patients who continued taking ELMIRON®, an additional 116/2499 (5%) of patients had improved pain scores. After 6 months, the percent of patients who reported the first onset of pain relief was less than 1.5% of patients who originally entered in the study (see Table 2). | |||
: [[File:{{PAGENAME}}02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--How Supplied--> | |||
|howSupplied= | |||
* ELMIRON® is supplied in white opaque hard gelatin capsules imprinted "BNP7600" containing 100 mg pentosan polysulfate sodium. Supplied in bottles of 100 capsules. | |||
:*NDC NUMBER 50458-098-01 | |||
*Storage | |||
:*Store at controlled room temperature 15°–30°C (59°–86°F). | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo= | |||
*Patients should take the drug as prescribed, in the dosage prescribed, and no more frequently than prescribed. Patients should be reminded that ELMIRON® has a weak anticoagulant effect. This effect may increase bleeding times. | |||
=====What is the most important information I should know about ELMIRON®?===== | |||
*ELMIRON® (pronounced EL ma ron) is used to treat the pain or discomfort of interstitial cystitis (IC). | |||
*You must take ELMIRON® as prescribed by your doctor in the dosage prescribed but no more frequently than prescribed. | |||
*ELMIRON® is a weak anticoagulant (blood thinner) which may increase bleeding. | |||
*Call your doctor if you will be undergoing surgery or will begin taking anticoagulant therapy such as [[warfarin]] sodium, [[heparin]], high doses of [[aspirin]], or anti-inflammatory drugs such as [[ibuprofen]]. | |||
=====What is ELMIRON®?===== | |||
*ELMIRON® is used to treat the pain or discomfort of [[interstitial cystitis]] (IC). It is not known exactly how ELMIRON® works, but it is not a pain medication like aspirin or acetaminophen and therefore must be taken continuously for relief as prescribed. | |||
=====Who should not take ELMIRON®?===== | |||
*Patients undergoing surgery should speak with their doctor about when to discontinue ELMIRON® prior to surgery. | |||
*ELMIRON® should be used during pregnancy only if clearly needed. | |||
=====What does your doctor need to know?===== | |||
*If you are taking anticoagulant therapy such as warfarin sodium, heparin, high doses of aspirin, or anti-inflammatory drugs such as ibuprofen. | |||
*If you are pregnant. | |||
*If you have any liver problems. | |||
=====How should I take ELMIRON®?===== | |||
*You should take 1 capsule of ELMIRON® by mouth three times a day, with water at least 1 hour before meals or 2 hours after meals. Each capsule contains 100 mg of ELMIRON®. | |||
=====What should I avoid while taking ELMIRON®?===== | |||
*Anticoagulant therapy such as warfarin sodium, heparin, high doses of aspirin or anti-inflammatory drugs such as ibuprofen until you speak with your doctor. | |||
=====What are the most common side effects of ELMIRON®?===== | |||
*The most common side effects are [[hair loss]], [[diarrhea]], [[nausea]], blood in the stool, [[headache]], [[rash]], upset stomach, abnormal liver function tests, [[dizziness]] and [[bruising]]. | |||
*Call your doctor if these side effects persist or are bothersome or if there is blood in your stool. | |||
*If you suspect that someone may have taken more than the prescribed dose of this medicine, contact your local poison control center or emergency room immediately. This medication was prescribed for your particular condition. Do not use it for another condition or give the drug to others. | |||
*This leaflet provides a summary of information about ELMIRON®. Medicines are sometimes prescribed for uses other than those listed in a Patient Leaflet. If you have any questions or concerns, or want more information about ELMIRON®, contact your doctor or pharmacist. Your pharmacist also has a longer leaflet about ELMIRON® that is written for health professionals that you can ask to read. | |||
<!--Precautions with Alcohol--> | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* Elmiron®<ref>{{Cite web | title = ELMIRON (pentosan polysulfate sodium) capsule, gelatin coated [Janssen Pharmaceuticals, Inc.] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f0ba651e-3d8a-11df-8fbe-119855d89593 }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike= | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
{{PillImage | |||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | |||
<!--Label Display Image--> | |||
{{LabelImage | |||
|fileName={{PAGENAME}}04.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}05.png|This image is provided by the National Library of Medicine. | |||
}} | |||
<!--Category--> | |||
[[Category:Drug]] | |||
Latest revision as of 17:15, 4 September 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pentosan polysulfate is a cystitis agent that is FDA approved for the {{{indicationType}}} of interstitial cystitis, chronic, relief of bladder pain or discomfort. Common adverse reactions include alopecia, rash, abdominal pain, diarrhea, indigestion, nausea, increased liver enzymes, dizziness, headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Interstitial cystitis, chronic, relief of bladder pain or discomfort
- Dosing Information
- ELMIRON® (pentosan polysulfate sodium) is indicated for the relief of bladder pain or discomfort associated with interstitial cystitis.
- The recommended dose of ELMIRON® is 300 mg/day taken as one 100 mg capsule orally three times daily. The capsules should be taken with water at least 1 hour before meals or 2 hours after meals.
- Patients receiving ELMIRON® should be reassessed after 3 months. If improvement has not occurred and if limiting adverse events are not present, ELMIRON® may be continued for another 3 months.
- The clinical value and risks of continued treatment in patients whose pain has not improved by 6 months is not known.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pentosan polysulfate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pentosan polysulfate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Pentosan polysulfate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pentosan polysulfate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pentosan polysulfate in pediatric patients.
Contraindications
- ELMIRON® is contraindicated in patients with known hypersensitivity to the drug, structurally related compounds, or excipients.
Warnings
- None.
Precautions
- General
- ELMIRON® is a weak anticoagulant (1/15 the activity of heparin). At a daily dose of 300 mg (n = 128), rectal hemorrhage was reported as an adverse event in 6.3% of patients. Bleeding complications of ecchymosis, epistaxis, and gum hemorrhage have been reported. Patients undergoing invasive procedures or having signs/symptoms of underlying coagulopathy or other increased risk of bleeding (due to other therapies such as coumarin anticoagulants, heparin, t-PA, streptokinase, high dose aspirin, or nonsteroidal anti-inflammatory drugs) should be evaluated for hemorrhage. Patients with diseases such as aneurysms, thrombocytopenia, hemophilia, gastrointestinal ulcerations, polyps, or diverticula should be carefully evaluated before starting ELMIRON®.
- A similar product that was given subcutaneously, sublingually, or intramuscularly (and not initially metabolized by the liver) is associated with delayed immunoallergic thrombocytopenia with symptoms of thrombosis and hemorrhage. Caution should be exercised when using ELMIRON® in patients who have a history of heparin induced thrombocytopenia.
- Alopecia is associated with pentosan polysulfate and with heparin products. In clinical trials of ELMIRON®, alopecia began within the first 4 weeks of treatment. Ninety-seven percent (97%) of the cases of alopecia reported were alopecia areata, limited to a single area on the scalp.
- Hepatic Insufficiency
- ELMIRON® has not been studied in patients with hepatic insufficiency. Because there is evidence of hepatic contribution to the elimination of ELMIRON®, hepatic impairment may have an impact on the pharmacokinetics of ELMIRON®. Caution should be exercised when using ELMIRON® in this patient population.
- Mildly (< 2.5 × normal) elevated transaminase, alkaline phosphatase, γ-glutamyl transpeptidase, and lactic dehydrogenase occurred in 1.2% of patients. The increases usually appeared 3 to 12 months after the start of ELMIRON® therapy, and were not associated with jaundice or other clinical signs or symptoms. These abnormalities are usually transient, may remain essentially unchanged, or may rarely progress with continued use. Increases in PTT and PT (< 1% for both) or thrombocytopenia (0.2%) were noted.
- Laboratory Test Findings
- Pentosan polysulfate sodium did not affect prothrombin time (PT) or partial thromboplastin time (PTT) up to 1200 mg per day in 24 healthy male subjects treated for 8 days. Pentosan polysulfate sodium also inhibits the generation of factor Xa in plasma and inhibits thrombin-induced platelet aggregation in human platelet rich plasma ex vivo.
Adverse Reactions
Clinical Trials Experience
- ELMIRON® was evaluated in clinical trials in a total of 2627 patients (2343 women, 262 men, 22 unknown) with a mean age of 47 [range 18 to 88 with 581 (22%) over 60 years of age]. Of the 2627 patients, 128 patients were in a 3–month trial and the remaining 2499 patients were in a long-term, unblinded trial.
- Deaths occurred in 6/2627 (0.2%) patients who received the drug over a period of 3 to 75 months. The deaths appear to be related to other concurrent illnesses or procedures, except in one patient for whom the cause was not known.
- Serious adverse events occurred in 33/2627 (1.3%) patients. Two patients had severe abdominal pain or diarrhea and dehydration that required hospitalization. Because there was not a control group of patients with interstitial cystitis who were concurrently evaluated, it is difficult to determine which events are associated with ELMIRON® and which events are associated with concurrent illness, medicine, or other factors.
- The adverse events described below were reported in an unblinded clinical trial of 2499 interstitial cystitis patients treated with ELMIRON®. Of the original 2499 patients, 1192 (48%) received ELMIRON® for 3 months; 892 (36%) received ELMIRON for 6 months; and 598 (24%) received ELMIRON® for one year, 355 (14%) received ELMIRON® for 2 years, and 145 (6%) for 4 years.
- Frequency (1 to 4%): Alopecia (4%), diarrhea (4%), nausea (4%), headache (3%), rash (3%), dyspepsia (2%), abdominal pain (2%), liver function abnormalities (1%), dizziness (1%).
- Frequency (≤ 1%):
- Digestive: Vomiting, mouth ulcer, colitis, esophagitis, gastritis, flatulence, constipation, anorexia, gum hemorrhage.
- Hematologic: Anemia, ecchymosis, increased prothrombin time, increased partial thromboplastin time, leukopenia, thrombocytopenia.
- Hypersensitive Reactions: Allergic reaction, photosensitivity.
- Respiratory System: Pharyngitis, rhinitis, epistaxis, dyspnea.
- Skin and Appendages: Pruritus, urticaria.
- Special Senses: Conjunctivitis, tinnitus, optic neuritis, amblyopia, retinal hemorrhage.
Postmarketing Experience
- ELMIRON® was evaluated in a randomized, double-blind, parallel group, Phase 4 study conducted in 380 patients with interstitial cystitis dosed for 32 weeks. At a daily dose of 300 mg (n = 128), rectal hemorrhage was reported as an adverse event in 6.3% of patients. The severity of the events was described as "mild" in most patients. Patients in that study who were administered ELMIRON® 900 mg daily, a dose higher than the approved dose, experienced a higher incidence of rectal hemorrhage, 15%.
- Liver Function Abnormality
- A randomized, double-blind, parallel group, phase 2 study was conducted in 100 men (51 ELMIRON® and 49 placebo) dosed for 16 weeks. At a daily dose of 900 mg, a dose higher than the approved dose, elevated liver function tests were reported as an adverse event in 11.8% (n = 6) of ELMIRON®-treated patients and 2% (n = 1) of placebo-treated patients.
Drug Interactions
- In a study in which healthy subjects received pentosan polysulfate sodium 100 mg capsule or placebo every 8 hours for 7 days, and were titrated with warfarin to an INR of 1.4 to 1.8, the pharmacokinetic parameters of R-warfarin and S-warfarin were similar in the absence and presence of pentosan polysulfate sodium. INR for warfarin + placebo and warfarin + pentosan polysulfate sodium were comparable.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Reproduction studies have been performed in mice and rats with intravenous daily doses of 15 mg/kg, and in rabbits with 7.5 mg/kg. These doses are 0.42 and 0.14 times the daily oral human doses of ELMIRON® when normalized to body surface area. These studies did not reveal evidence of impaired fertility or harm to the fetus from ELMIRON®. Direct in vitro bathing of cultured mouse embryos with pentosan polysulfate sodium (PPS) at a concentration of 1 mg/mL may cause reversible limb bud abnormalities. Adequate and well-controlled studies have not been performed in pregnant women. Because animal studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pentosan polysulfate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pentosan polysulfate during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ELMIRON® is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 16 years have not been established.
Geriatic Use
There is no FDA guidance on the use of Pentosan polysulfate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Pentosan polysulfate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pentosan polysulfate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pentosan polysulfate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pentosan polysulfate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pentosan polysulfate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pentosan polysulfate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Pentosan polysulfate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Pentosan polysulfate in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Overdose has not been reported. Based upon the pharmacodynamics of the drug, toxicity is likely to be reflected as anticoagulation, bleeding, thrombocytopenia, liver function abnormalities, and gastric distress. At a daily dose of 900 mg for 32 weeks (n = 127) in a clinical trial, rectal hemorrhage was reported as an adverse event in 15% of patients. At a daily dose of ELMIRON® 900 mg for 16 weeks in a clinical trial that enrolled 51 patients in the ELMIRON® group and 49 in the placebo group, elevated liver function tests were reported as an adverse event in 11.8% of patients in the ELMIRON® group and 2% of patients in the placebo group.
Management
- In the event of acute overdosage, the patient should be given gastric lavage if possible, carefully observed and given symptomatic and supportive treatment.
Chronic Overdose
There is limited information regarding Chronic Overdose of Pentosan polysulfate in the drug label.
Pharmacology
| |
Pentosan polysulfate
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | (free acid) 116001-96-8 (sodium salt) |
| ATC code | C05 G04BX15 (WHO) Template:ATCvet |
| PubChem | |
| Chemical data | |
| Formula | (C5H6Na2O10S2)n |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | Urine |
| Therapeutic considerations | |
| Pregnancy cat. |
B |
| Legal status | |
| Routes | Oral, intramuscular, intra-articular, intraventricular |
Mechanism of Action
- Pentosan polysulfate sodium is a low molecular weight heparin-like compound. It has anticoagulant and fibrinolytic effects. The mechanism of action of pentosan polysulfate sodium in interstitial cystitis is not known.
Structure
- Pentosan polysulfate sodium is a semi-synthetically produced heparin-like macromolecular carbohydrate derivative, which chemically and structurally resembles glycosaminoglycans. It is a white odorless powder, slightly hygroscopic and soluble in water to 50% at pH 6. It has a molecular weight of 4000 to 6000 Dalton with the following structural formula:
- ELMIRON® is supplied in white opaque hard gelatin capsules containing 100 mg pentosan polysulfate sodium, microcrystalline cellulose, and magnesium stearate. It also contains pharmaceutical glaze (modified) in SD-45, synthetic black iron oxide, FD&C Blue No. 2 aluminum lake, FD&C Red No. 40 aluminum lake, FD&C Blue No. 1 aluminum lake, D&C Yellow No. 10 aluminum lake, n-butyl alcohol, propylene glycol, SDA-3A alcohol, and titanium dioxide. It is formulated for oral use.
Pharmacodynamics
- The mechanism by which pentosan polysulfate sodium achieves its effects in patients is unknown. In preliminary clinical models, pentosan polysulfate sodium adhered to the bladder wall mucosal membrane. The drug may act as a buffer to control cell permeability preventing irritating solutes in the urine from reaching the cells.
Pharmacokinetics
- Absorption
- In a clinical pharmacology study in which healthy female volunteers received a single oral 300 or 450 mg dose of pentosan polysulfate sodium containing radiolabeled drug as a solution under fasted conditions, maximal levels of plasma radioactivity were seen approximately at a median of 2 hours (range 0.6-120 hours) after dosing. Based on urinary excretion of radioactivity, a mean of approximately 6% of a radiolabeled oral dose of pentosan polysulfate sodium is absorbed and reaches the systemic circulation.
- Food Effects
- In clinical trials, ELMIRON® was administered with water 1 hour before or 2 hours after meals; the effect of food on absorption of pentosan polysulfate sodium is not known.
- Distribution
- Preclinical studies with parenterally administered radiolabeled pentosan polysulfate sodium showed distribution to the uroepithelium of the genitourinary tract with lesser amounts found in the liver, spleen, lung, skin, periosteum, and bone marrow. Erythrocyte penetration is low in animals.
- Metabolism
- The fraction of pentosan polysulfate sodium that is absorbed is metabolized by partial desulfation in the liver and spleen, and by partial depolymerization in the kidney to a large number of metabolites. Both the desulfation and depolymerization can be saturated with continued dosing.
- Excretion
- Following administration of an oral solution of a 300 or 450 mg dose of pentosan polysulfate sodium containing radiolabeled drug to groups of healthy subjects, plasma radioactivity declined with mean half-lives of 27 and 20 hours, respectively. A large proportion of the orally administered dose of pentosan polysulfate sodium (mean 84% in the 300 mg group and 58% in the 450 mg group) is excreted in feces as unchanged drug. A mean of 6% of an oral dose is excreted in the urine, mostly as desulfated and depolymerized metabolites. Only a small fraction of the administered dose (mean 0.14%) is recovered as intact drug in urine.
- Special Populations
- The pharmacokinetics of pentosan polysulfate sodium has not been studied in geriatric patients or in patients with hepatic or renal impairment.
- Drug-Drug Interactions
- In a study in which healthy subjects received pentosan polysulfate sodium 100 mg capsule or placebo every 8 hours for 7 days, and were titrated with warfarin to an INR of 1.4 to 1.8, the pharmacokinetic parameters of R-warfarin and S-warfarin were similar in the absence and presence of pentosan polysulfate sodium. INR for warfarin + placebo and warfarin + pentosan polysulfate sodium were comparable.
Nonclinical Toxicology
Carcinogenicity, Mutagenesis, Impairment of Fertility
- Long term carcinogenicity studies of ELMIRON® in F344/N rats and B6C3F1 mice have been conducted. In these studies, ELMIRON® was orally administered once daily via gavage, 5 days per week, for up to 2 years. The dosages administered to mice were 56, 168 or 504 mg/kg. The dosages administered to rats were 14, 42, or 126 mg/kg for males, and 28, 84, or 252 mg/kg for females. The dosages tested were up to 60 times the maximum recommended human dose (MRHD) in rats, and up to 117 times the MRHD in mice, on a mg/kg basis. The results of these studies in rodents showed no clear evidence of drug-related tumorigenesis or carcinogenic risk.
- Pentosan polysulfate sodium was not clastogenic or mutagenic when tested in the mouse micronucleus test or the Ames test (S. typhimurium). The effect of pentosan polysulfate sodium on spermatogenesis has not been investigated.
Clinical Studies
- ELMIRON® was evaluated in two clinical trials for the relief of pain in patients with chronic interstitial cystitis (IC). All patients met the NIH definition of IC based upon the results of cystoscopy, cytology, and biopsy. One blinded, randomized, placebo-controlled study evaluated 151 patients (145 women, 5 men, 1 unknown) with a mean age of 44 years (range 18 to 81). Approximately equal numbers of patients received either placebo or ELMIRON® 100 mg three times a day for 3 months. Clinical improvement in bladder pain was based upon the patient's own assessment. In this study, 28/74 (38%) of patients who received ELMIRON® and 13/74 (18%) of patients who received placebo showed greater than 50% improvement in bladder pain (p = 0.005).
- A second clinical trial, the physician's usage study, was a prospectively designed retrospective analysis of 2499 patients who received ELMIRON® 300 mg a day without blinding. Of the 2499 patients, 2220 were women, 254 were men, and 25 were of unknown sex. The patients had a mean age of 47 years and 23% were over 60 years of age. By 3 months, 1307 (52%) of the patients had dropped out or were ineligible for analysis, overall, 1192 (48%) received ELMIRON® for 3 months; 892 (36%) received ELMIRON® for 6 months; and 598 (24%) received ELMIRON® for one year.
- Patients had unblinded evaluations every 3 months for the patient's rating of overall change in pain in comparison to baseline and for the difference calculated in "pain/discomfort" scores. At baseline, pain/discomfort scores for the original 2499 patients were severe or unbearable in 60%, moderate in 33% and mild or none in 7% of patients. The extent of the patients' pain improvement is shown in Table 1.
- At 3 months, 722/2499 (29%) of the patients originally in the study had pain scores that improved by one or two categories. By 6 months, in the 892 patients who continued taking ELMIRON®, an additional 116/2499 (5%) of patients had improved pain scores. After 6 months, the percent of patients who reported the first onset of pain relief was less than 1.5% of patients who originally entered in the study (see Table 2).
How Supplied
- ELMIRON® is supplied in white opaque hard gelatin capsules imprinted "BNP7600" containing 100 mg pentosan polysulfate sodium. Supplied in bottles of 100 capsules.
- NDC NUMBER 50458-098-01
- Storage
- Store at controlled room temperature 15°–30°C (59°–86°F).
Storage
There is limited information regarding Pentosan polysulfate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Pentosan polysulfate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pentosan polysulfate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should take the drug as prescribed, in the dosage prescribed, and no more frequently than prescribed. Patients should be reminded that ELMIRON® has a weak anticoagulant effect. This effect may increase bleeding times.
What is the most important information I should know about ELMIRON®?
- ELMIRON® (pronounced EL ma ron) is used to treat the pain or discomfort of interstitial cystitis (IC).
- You must take ELMIRON® as prescribed by your doctor in the dosage prescribed but no more frequently than prescribed.
- ELMIRON® is a weak anticoagulant (blood thinner) which may increase bleeding.
- Call your doctor if you will be undergoing surgery or will begin taking anticoagulant therapy such as warfarin sodium, heparin, high doses of aspirin, or anti-inflammatory drugs such as ibuprofen.
What is ELMIRON®?
- ELMIRON® is used to treat the pain or discomfort of interstitial cystitis (IC). It is not known exactly how ELMIRON® works, but it is not a pain medication like aspirin or acetaminophen and therefore must be taken continuously for relief as prescribed.
Who should not take ELMIRON®?
- Patients undergoing surgery should speak with their doctor about when to discontinue ELMIRON® prior to surgery.
- ELMIRON® should be used during pregnancy only if clearly needed.
What does your doctor need to know?
- If you are taking anticoagulant therapy such as warfarin sodium, heparin, high doses of aspirin, or anti-inflammatory drugs such as ibuprofen.
- If you are pregnant.
- If you have any liver problems.
How should I take ELMIRON®?
- You should take 1 capsule of ELMIRON® by mouth three times a day, with water at least 1 hour before meals or 2 hours after meals. Each capsule contains 100 mg of ELMIRON®.
What should I avoid while taking ELMIRON®?
- Anticoagulant therapy such as warfarin sodium, heparin, high doses of aspirin or anti-inflammatory drugs such as ibuprofen until you speak with your doctor.
What are the most common side effects of ELMIRON®?
- The most common side effects are hair loss, diarrhea, nausea, blood in the stool, headache, rash, upset stomach, abnormal liver function tests, dizziness and bruising.
- Call your doctor if these side effects persist or are bothersome or if there is blood in your stool.
- If you suspect that someone may have taken more than the prescribed dose of this medicine, contact your local poison control center or emergency room immediately. This medication was prescribed for your particular condition. Do not use it for another condition or give the drug to others.
- This leaflet provides a summary of information about ELMIRON®. Medicines are sometimes prescribed for uses other than those listed in a Patient Leaflet. If you have any questions or concerns, or want more information about ELMIRON®, contact your doctor or pharmacist. Your pharmacist also has a longer leaflet about ELMIRON® that is written for health professionals that you can ask to read.
Precautions with Alcohol
- Alcohol-Pentosan polysulfate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Elmiron®[1]
Look-Alike Drug Names
There is limited information regarding Pentosan polysulfate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Pentosan polysulfate |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Pentosan polysulfate |Label Name=Pentosan polysulfate04.png
}}
{{#subobject:
|Label Page=Pentosan polysulfate |Label Name=Pentosan polysulfate05.png
}}