Moxifloxacin dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
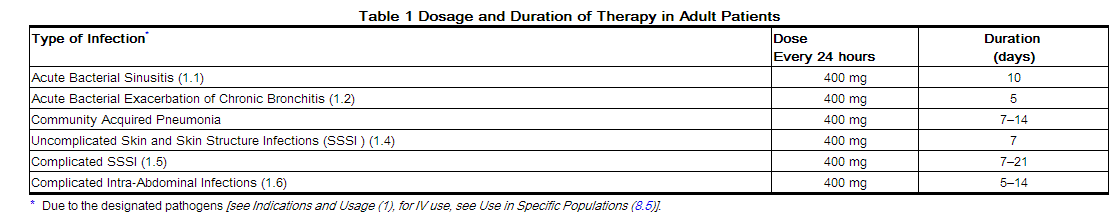
DOSAGE AND ADMINISTRATION
Dosage in Adult Patients
The dose of AVELOX is 400 mg (orally or as an intravenous infusion) once every 24 hours. The duration of therapy depends on the type of infection as described in Table 1.
Intravenous formulation is indicated when it offers a route of administration advantageous to the patient (for example, patient cannot tolerate an oral dosage form). When switching from intravenous to oral formulation, no dosage adjustment is necessary. Patients whose therapy is started with AVELOX IV may be switched to AVELOX Tablets when clinically indicated at the discretion of the physician.
Drug Interactions with Multivalent Cations
Oral doses of AVELOX should be administered at least 4 hours before or 8 hours after products containing magnesium, aluminum, iron or zinc, including antacids, sucralfate, multivitamins and VIDEX® (didanosine) chewable/buffered tablets or the pediatric powder for oral solution [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Administration Instructions
AVELOX Film-Coated Tablets
AVELOX Tablets can be taken with or without food, drink fluids liberally.
AVELOX IV Solution for Infusion
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
AVELOX IV should be administered by INTRAVENOUS infusion only. It is not intended for intra-arterial, intramuscular, intrathecal, intraperitoneal, or subcutaneous administration.
AVELOX IV should be administered by intravenous infusion over a period of 60 minutes by direct infusion or through a Y-type intravenous infusion set which may already be in place. Caution: rapid or bolus intravenous infusion must be avoided.
Because only limited data are available on the compatibility of AVELOX intravenous injection with other intravenous substances, additives or other medications should not be added to AVELOX IV or infused simultaneously through the same intravenous line. If the same intravenous line or a Y-type line is used for sequential infusion of other drugs, or if the “piggyback” method of administration is used, the line should be flushed before and after infusion of AVELOX IV with an infusion solution compatible with AVELOX IV as well as with other drug(s) administered via this common line.
Preparation for Administration of AVELOX IV
To prepare AVELOX IV injection premix in flexible containers:
1. Close flow control clamp of administration set. 2. Remove cover from port at bottom of container. 3. Insert piercing pin from an appropriate transfer set (for example, one that does not require excessive force, such as ISO compatible administration set) into port with a gentle twisting motion until pin is firmly seated.
NOTE: Refer to complete directions that have been provided with the administration set.
DOSAGE FORMS AND STRENGTHS
AVELOX Tablets
• Containing moxifloxacin hydrochloride (equivalent to 400 mg moxifloxacin) • Oblong, dull red film-coated tablets • Imprinted with BAYER on one side and M400 on the other
AVELOX IV
• Containing 400 mg moxifloxacin in 0.8% saline (moxifloxacin hydrochloride in sodium chloride injection) with pH ranging from 4.1 to 4.6. • Ready-to-use 250 mL latex-free flexibags. No further dilution is necessary • Sterile, preservative free, 0.8% sodium chloride aqueous solution of moxifloxacin hydrochloride
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21277slr018,21085slr023_avelox_lbl.pdf