Moxifloxacin description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
DESCRIPTION
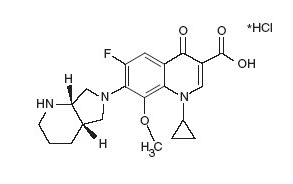
AVELOX (moxifloxacin) hydrochloride is a synthetic broad spectrum antibacterial agent for oral and intravenous administration. Moxifloxacin, a fluoroquinolone, is available as the monohydrochloride salt of 1-cyclopropyl-7-[(S,S)-2,8-diazabicyclo[4.3.0]non-8-yl]-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3 quinoline carboxylic acid. It is a slightly yellow to yellow crystalline substance with a molecular weight of 437.9. Its empirical formula is C21H24FN3O4*HCl and its chemical structure is as follows:
AVELOX Tablets
AVELOX Tablets are available as film-coated tablets containing moxifloxacin hydrochloride (equivalent to 400 mg moxifloxacin). • The inactive ingredients are microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, magnesium stearate, hypromellose, titanium dioxide, polyethylene glycol and ferric oxide.
AVELOX IV
• AVELOX IV is available in ready-to-use 250 mL latex-free flexibags as a sterile, preservative free, 0.8% sodium chloride aqueous solution of moxifloxacin hydrochloride (containing 400 mg moxifloxacin) with pH ranging from 4.1 to 4.6. • The appearance of the intravenous solution is yellow. The color does not affect, nor is it indicative of, product stability. • The inactive ingredients are sodium chloride, USP, Water for Injection, USP, and may include hydrochloric acid and/or sodium hydroxide for pH adjustment. • AVELOX IV contains approximately 34.2 mEq (787 mg) of sodium in 250 mL
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21277slr018,21085slr023_avelox_lbl.pdf