Moxifloxacin adverse reactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
ADVERSE REACTIONS
Serious and Otherwise Important Adverse Reactions
The following serious and otherwise important adverse reactions are discussed in greater detail in the warnings and precautions section of the label:
• Tendinopathy and Tendon Rupture [see Warnings and Precautions (5.1)] • QT Prolongation [see Warnings and Precautions (5.3)] • Hypersensitivity Reactions [see Warnings and Precautions (5.4)] • Other Serious and Sometimes Fatal Reactions [see Warnings and Precautions (5.5)] • Central Nervous System Effects [see Warnings and Precautions (5.6)] • Clostridium difficile-Associated Diarrhea [see Warnings and Precautions (5.7)] • Peripheral Neuropathy that may be irreversible [see Warnings and Precautions (5.8)] • Photosensitivity/Phototoxicity [see Warnings and Precautions (5.10)] • Development of Drug Resistant Bacteria [see Warnings and Precautions (5.11)]
Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to AVELOX in 14981 patients in 71 active controlled Phase II- IV clinical trials in different indications [see Indications and Usage (1)]. The population studied had a mean age of 50 years (approximately 73% of the population was <65 years of age), 50% were male, 63% were Caucasian, 12% were Asian and 9% were Black. Patients received AVELOX 400 mg once daily PO, IV, or sequentially (IV followed by PO). Treatment duration was usually 6-10 days, and the mean number of days on therapy was 9 days.
Discontinuation of AVELOX due to adverse events occurred in 5.0% of patients overall, 4.1% of patients treated with 400 mg PO, 3.9% with 400 mg IV and 8.2% with sequential therapy 400 mg PO/IV. The most common adverse events leading to discontinuation with the 400 mg PO doses were nausea (0.8%), diarrhea (0.5%), dizziness (0.5%), and vomiting (0.4%). The most common adverse event leading to discontinuation with the 400 mg IV dose was rash (0.5%). The most common adverse events leading to discontinuation with the 400 mg IV/PO sequential dose were diarrhea (0.5%), pyrexia (0.4%).
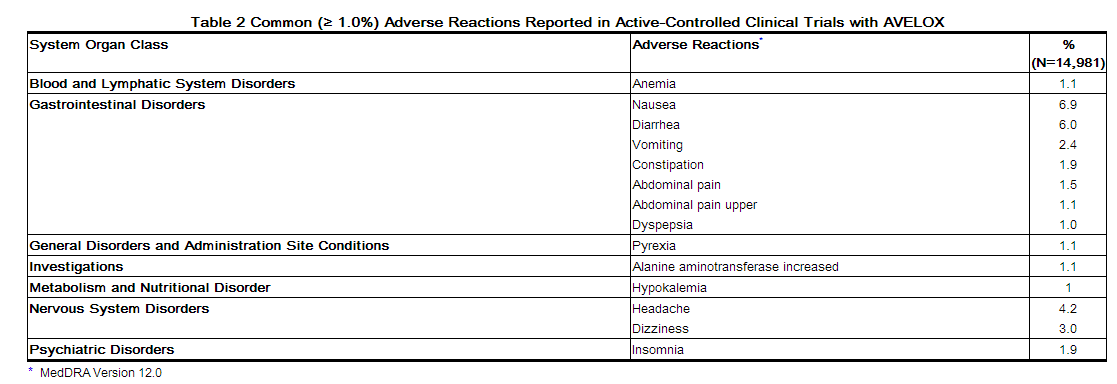
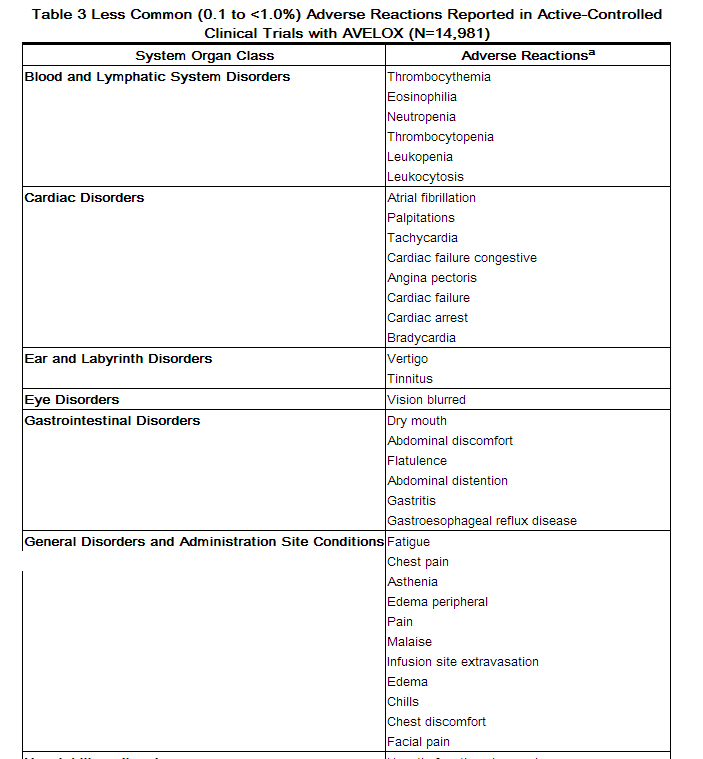
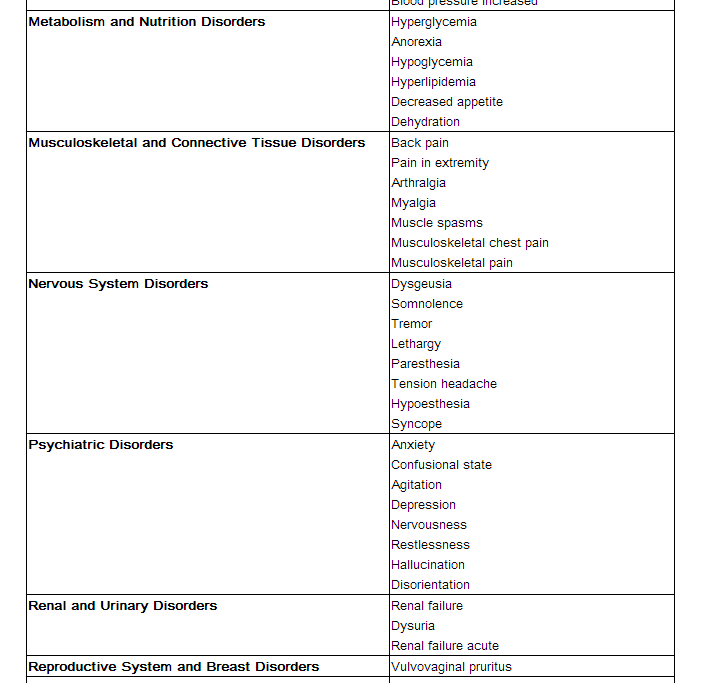
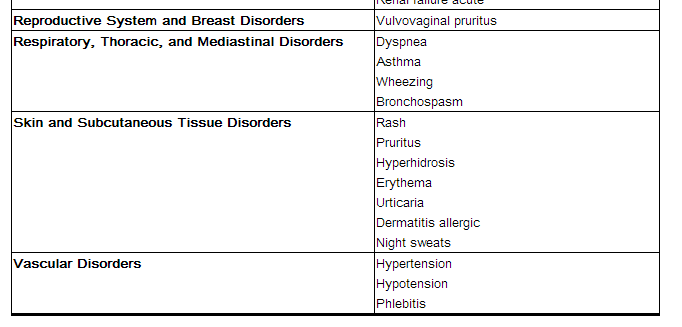
Adverse reactions occurring in ≥1% of AVELOX-treated patients and less common adverse reactions, occurring in 0.1 to <1% of AVELOX-treated patients, are shown in Tables 2 and Table 3, respectively. The most common adverse drug reactions (≥3%) are nausea, diarrhea, headache, and dizziness.
MedDRA Version 12.0
Laboratory Changes
Changes in laboratory parameters, without regard to drug relationship, which are not listed above and which occurred in ≥ 2% of patients and at an incidence greater than in controls included: increases in MCH, neutrophils, WBCs, PT ratio, ionized calcium, chloride, albumin, globulin, bilirubin; decreases in hemoglobin, RBCs, neutrophils, eosinophils, basophils, PT ratio, glucose, pO2, bilirubin, and amylase. It cannot be determined if any of the above laboratory abnormalities were caused by the drug or the underlying condition being treated.
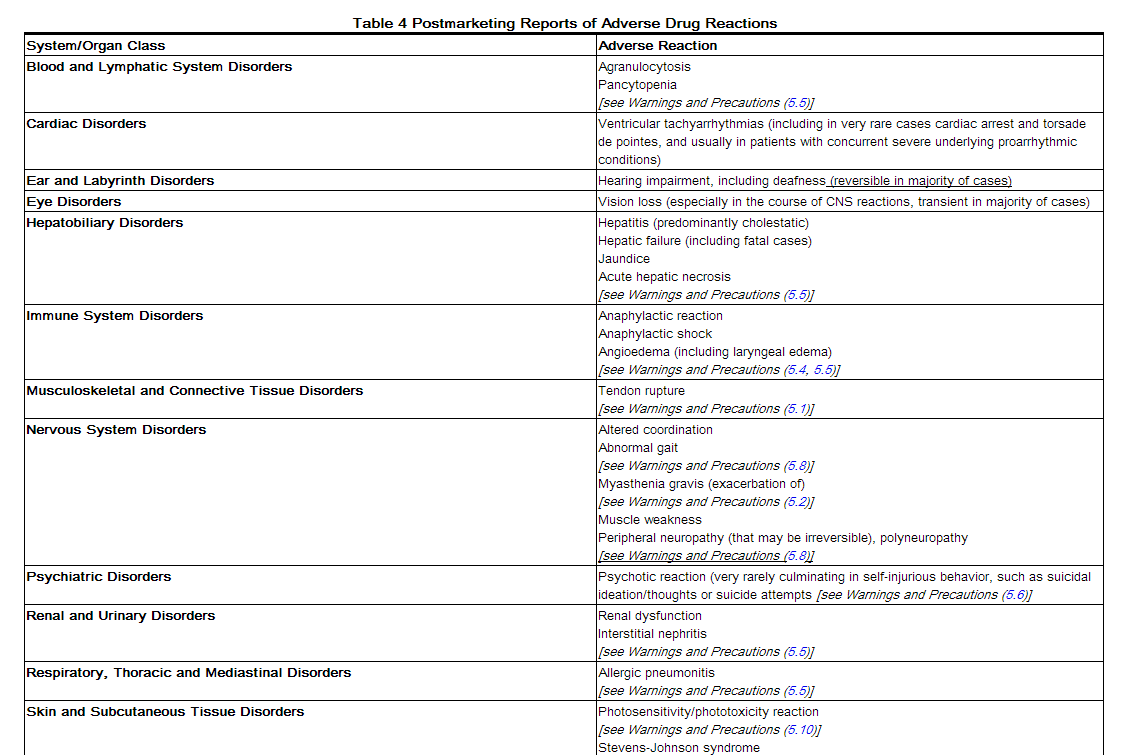
Postmarketing Experience
Table 4 lists adverse reactions that have been identified during post-approval use of AVELOX. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21277slr018,21085slr023_avelox_lbl.pdf