Moxidectin: Difference between revisions
m (Protected "Moxidectin": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| Line 1: | Line 1: | ||
{| | {{DrugProjectFormSinglePage | ||
|- | |authorTag={{ZL}} | ||
| | |aOrAn=an | ||
[[ | |genericName=Moxidectin | ||
|drugClass=[[anthelmintic]] | |||
|- | |indication=[[onchocerciasis]] due to Onchocerca volvulus in patients aged 12 years and older | ||
| | |indicationType=treatment | ||
|hasBlackBoxWarning= | |||
|blackBoxWarningTitle= | |||
|blackBoxWarningBody= | |||
|fdaLIADAdult= | |||
Indication | |||
| | *Moxidectin Tablets are indicated for the treatment of [[onchocerciasis]] due to Onchocerca volvulus in patients aged 12 years and older. | ||
Limitations of Use | |||
*Moxidectin Tablets do not kill adult O. volvulus. Follow-up evaluation is advised. | |||
*The safety and efficacy of repeat administration of Moxidectin Tablets in patients with O. volvulus has not been studied. | |||
Dosage | |||
| | *The recommended dosage of Moxidectin Tablet is a single dose of 8 mg (four 2 mg tablets) taken orally with or without food. | ||
| | |offLabelAdultGuideSupport= | ||
| | There is limited information regarding moxidectin Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label. | ||
|offLabelAdultNoGuideSupport= | |||
| | There is limited information regarding moxidectin Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label. | ||
| | |fdaLIADPed= | ||
| | Indication | ||
| | *Moxidectin Tablets are indicated for the treatment of [[onchocerciasis]] due to Onchocerca volvulus in patients aged 12 years and older. | ||
| | *The safety and effectiveness of Moxidectin Tablets in pediatric patients under 12 years of age has not been established. | ||
Limitations of Use | |||
*Moxidectin Tablets do not kill adult O. volvulus. Follow-up evaluation is advised. | |||
| | *The safety and efficacy of repeat administration of Moxidectin Tablets in patients with O. volvulus has not been studied. | ||
| | Dosage | ||
| | *The recommended dosage of Moxidectin Tablet for pediatric patients 12 years and older is a single dose of 8 mg (four 2 mg tablets) taken orally with or without food. | ||
| | |offLabelPedGuideSupport= | ||
There is limited information regarding moxidectin Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label. | |||
|offLabelPedNoGuideSupport= | |||
| | There is limited information regarding moxidectin Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label. | ||
| | |contraindications= | ||
| | None. | ||
| | |warnings= | ||
| | =====Cutaneous, Ophthalmological and/or Systemic Adverse Reactions===== | ||
| | *Treatment with Moxidectin Tablets may cause [[cutaneous]], [[ophthalmological]] and/or systemic reactions of varying severity (Mazzotti reaction). These adverse reactions are due to allergic and inflammatory host responses to the death of microfilariae. There is a trend toward an increased incidence of these adverse reactions in patients with higher microfilarial burden. | ||
| | *The clinical manifestations of Mazzotti reaction includes [[pruritus]], [[headache]], [[pyrexia]], [[rash]], [[urticaria]], [[hypotension]] (including symptomatic orthostatic hypotension and dizziness), [[tachycardia]], [[edema]], [[lymphadenopathy]], [[arthralgia]], [[myalgia]], [[chills]], [[paresthesia]] and [[asthenia]]. Ophthalmological manifestations include [[conjunctivitis]], [[eye pain]], eye pruritus, eyelid swelling, blurred vision, [[photophobia]], changes in visual acuity, [[hyperemia]], ocular discomfort and watery eyes. These adverse reactions generally occur and resolve in the first week post-treatment. Laboratory changes include [[eosinophilia]], [[eosinopenia]], [[lymphocytopenia]], [[neutropenia]], and increases in alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT) and lactate dehydrogenase (LDH). [[Proteinuria]] has also been reported. | ||
| | *Treatment of severe Mazzotti reactions has not been evaluated in controlled clinical trials. Symptomatic treatments such as oral hydration, recumbency, intravenous normal saline, and/or parenteral [[corticosteroids]] have been used to treat orthostatic hypotension. [[Antihistamines]] and/or [[analgesics]] have been used for most mild to moderate cases. | ||
| | =====Symptomatic Orthostatic Hypotension===== | ||
*An increased number of patients who received Moxidectin Tablets developed symptomatic orthostatic hypotension with inability to stand without support after lying down for 5 minutes (in an orthostatic hypotension provocation test); 47/978 (5%) compared with 8/494 (2%) who received [[ivermectin]]. The decreases in blood pressure were transient, managed by resumption of recumbency and most commonly occurred on Days 1 and 2 post-treatment. Advise patients that if they feel dizzy or light-headed after taking Moxidectin Tablets, they should lie down until the symptoms resolve. | |||
=====Encephalopathy in Loa loa Co-infected Patients===== | |||
*Patients with [[onchocerciasis]] who are also infected with [[Loa loa]] may develop a serious or even fatal | |||
encephalopathy following treatment with Moxidectin Tablets. | |||
*Moxidectin Tablets have not been studied in patients co-infected with [[Loa loa]]. Therefore, it is recommended that individuals who warrant treatment with Moxidectin Tablets and have had exposure to Loa loa-endemic areas undergo diagnostic screening for loiasis prior to treatment. | |||
=====Edema and Worsening of Onchodermatitis===== | |||
*Patients with hyper-reactive onchodermatitis (sowda) may be more likely than others to experience severe [[edema]] and worsening of onchodermatitis following the use of Moxidectin Tablets. Symptomatic treatment has been used to manage patients who have experienced edema and worsening of onchodermatitis. | |||
|adverseReactions=[[eosinophilia]], [[pruritus]], musculoskeletal pain, [[headache]], [[lymphopenia]], [[tachycardia]], [[rash]], [[abdominal pain]], [[hypotension]], [[pyrexia]], [[leukocytosis]], [[influenza]]-like illness, [[neutropenia]], [[cough]], lymph node pain, [[dizziness]], [[diarrhea]], [[hyponatremia]] and peripheral swelling | |||
|clinicalTrials= | |||
*Because clinical trials are conducted under varying controlled conditions, adverse reaction rates observed in one clinical trial cannot be directly compared to rates observed in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | |||
*The safety of Moxidectin Tablets was evaluated in two randomized, double-blind, active-controlled studies (Trial 1 and Trial 2) [see Clinical Studies]. In Trial 1, 978 patients received Moxidectin Tablets as a single oral dose of 8 mg and 494 patients received ivermectin as a single oral dose of approximately 150 mcg/kg. In Trial 2, 127 patients received Moxidectin Tablets as a single oral dose ranging from 2 mg (this is not an approved dose) to 8 mg (38 received the recommended 8 mg dose) and 45 patients received ivermectin as a single oral dose of approximately 150 mcg/kg. | |||
Most Common Adverse Reactions | |||
*No patients withdrew from either trial due to adverse reactions. Adverse Reactions reported in Trial 1 in > 10% of patients are summarized in Table 1. Most were related to physical, vital signs and laboratory changes associated with Mazzotti reaction. | |||
[[File:MoxidectinTable1.1.png|600px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clear}} | |||
[[File:MoxidectinTable1.2.png|600px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clear}} | |||
*The most common adverse reactions in patients (n = 38) treated with 8 mg moxidectin in Trial 2 were similar to the adverse reactions noted in Trial 1 described in Table 1 above. | |||
Other Adverse Reactions Reported in Clinical Trials | |||
*The following adverse reactions occurred in less than 10% of subjects receiving Moxidectin Tablets in Trial 1: | |||
*Ocular Adverse Reactions: In Trial 1, the most common ocular adverse reactions (occurring in ≥ 0.5% of patients) are shown in Table 2. | |||
[[File:MoxidectinTable2.png|600px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clear}} | |||
Hepatobiliary Adverse Reactions | |||
*More patients in the moxidectin arm experienced elevation in bilirubin above the upper limit of normal and elevation in transaminases > 5x upper limit of normal. Twenty-seven (2.8%) patients in the moxidectin arm and 3 (0.6%) patients in the [[ivermectin]] arm had [[hyperbilirubinemia]]. Most of the patients had single measurements of hyperbilirubinemia without concurrent elevation in transaminases. | |||
*Nine (1%) patients in the moxidectin arm and 2 (0.4%) patients in the ivermectin arm had elevation in ALT of more than 5x upper limit of normal; ten (1%) patients in the moxidectin arm and 3 (0.6%) patients in the ivermectin arm had elevation in AST to more than 5x upper limit of normal. | |||
Laboratory Abnormalities | |||
*Laboratory abnormalities occurring in at least 1% of patients in the Trial 1 are described in Table 3. | |||
[[File:MoxidectinTable3.png|600px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clear}} | |||
|postmarketing= | |||
|drugInteractions= | |||
=====Midazolam (CYP3A4 substrate)===== | |||
*In healthy subjects, concomitant administration of a single 8 mg oral dose of Moxidectin Tablets did not have an effect on the pharmacokinetics of [[midazolam]]. Moxidectin can be co-administered with CYP3A4 substrates. | |||
|FDAPregCat= | |||
|useInPregnancyFDA= | |||
Risk Summary | |||
*Limited available data on the use of Moxidectin Tablets in pregnant women are insufficient to establish whether there is a moxidectin-associated risk for major [[birth defects]] and [[miscarriage]]. Moxidectin administered orally to pregnant rats during the period of organogenesis (Gestation Days (GD) 6 to 15), was not associated with significant embryo-fetal developmental effects at doses of approximately 15 times the recommended human dose based on body surface area. When moxidectin was dosed orally to pregnant rabbits during the period of organogenesis (GD 7 - 19), no embryo-fetal developmental effects were observed at oral doses of moxidectin up to 24 times the recommended human dose based on body surface area. | |||
*Daily parental oral administration of dietary moxidectin to rats prior to mating, and through mating, gestation, and lactation was associated with decreased survival and body weights for first-generation offspring without maternal toxicity at moxidectin doses less than 2-times the recommended human dose based on body surface area comparison. Daily dietary moxidectin did not produce maternal toxicity or adverse effects for first- and second-generation offspring at doses approximately equivalent to the recommended human dose based on body surface area comparison. Offspring were assessed for survival, body weights, and [[fertility]]. Developmental milestones were not assessed in this study. | |||
*The estimated background risk of major [[birth defects]] and [[miscarriage]] for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. | |||
Animal Data | |||
*In a rat embryo-fetal development study, daily oral administration of moxidectin at 12 mg/kg/day (approximately 15 times the recommended human dose of 8 mg based on body surface area comparison) during Gestation Days (GDs) 6 to 15 significantly increased the fetal incidence, but not the litter incidence of cleft palate and the fetal and litter incidence of a skeletal variation, wavy ribs, at a maternally toxic dose. Mean maternal food consumption, body weights, and body weight gain were significantly decreased at moxidectin doses of 10 and 12 mg/kg/day compared to control values. The no observed adverse effect level (NOAEL) value for maternal and fetal toxicity was considered to be 5 and 10 mg/kg/day respectively (approximately 6 and 12 times, respectively, the recommended human dose based on body surface area comparison). In the rabbit, daily oral administration of moxidectin at ≥ 5 mg/kg/day from GD7 to GD19 was not associated with fetal weight loss or malformations but resulted in significantly decreased maternal food consumption and body weight gains. The NOAEL value for maternal and fetal toxicity in the rabbit was 1 mg/kg/day and 10 mg/kg/day respectively (approximately 2 times and 24 times, respectively, the recommended human dose based on body surface area comparison). In a pre-postnatal study in rats, parental oral administration of dietary moxidectin prior to mating, through mating, gestation, and lactation did not produce adverse effects in first-generation or second- generation offspring at a maternal NOAEL dose of 0.824 mg/kg/day (approximately equivalent to the recommended human dose based on body surface area comparison). However, at moxidectin doses | |||
≥ 1.1 mg/kg/day (approximately equivalent to 1.3 times the recommended human dose based on body surface area comparison), the survival and body weights of first-generation offspring were significantly decreased during the lactation period, and the number of live fetuses at birth was significantly decreased with a maternal moxidectin dose of 11 mg/kg/day (approximately equivalent to 13 times the recommended human dose based on body surface area comparison). In this study, offspring were assessed for survival, body weights, and fertility, and developmental milestones were not assessed. | |||
|AUSPregCat= | |||
|useInPregnancyAUS= | |||
|useInLaborDelivery= | |||
|useInNursing= | |||
Risk Summary | |||
*Moxidectin was detected in the milk of lactating women following a single 8 mg dose of Moxidectin Tablets [see Data]. There are no data on the effects of Moxidectin Tablets on the breast-fed infant or milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Moxidectin Tablets and any potential adverse effects on the breastfed child from Moxidectin Tablets or from the underlying maternal condition. | |||
Data | |||
*A pharmacokinetic study in twelve healthy adult lactating women who were 21 to 100 weeks post partum evaluated the concentrations of moxidectin in plasma and breast milk collected over a period of 28 days following a single 8 mg dose of Moxidectin Tablets. The mean (± SD) exposure ratio of moxidectin present in human breast milk to that of human plasma was approximately 1.77 (± 0.66) over a collection period of | |||
28 days. The estimated mean (± SD) total infant dose, assuming the infants would consume all the breast milk collected during the study, was 0.056 mg (± 0.024 mg), which would be approximately 0.70% (± 0.30%) of the maternal dose. The effects of moxidectin or its metabolites on the breast-fed child or milk production were not evaluated. | |||
|useInPed= | |||
*The safety and effectiveness of Moxidectin Tablets have been established in pediatric patients 12 years of age and older. In Trial 1, (n = 53 patients aged 12 to 17 years), the safety and effectiveness was similar to that observed in adults. The safety and effectiveness of Moxidectin Tablets in pediatric patients under 12 years of age has not been established. | |||
|useInGeri= | |||
*Of the total number of patients included in Trial 1 that were treated with Moxidectin Tablets, 83 were aged 65 and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | |||
|useInGender= | |||
|useInRace= | |||
|useInRenalImpair= | |||
*No dose adjustment of Moxidectin Tablets is necessary for patients with mild (creatinine clearance (CrCL) 60 to 89 mL/min) to moderate (CrCL 30 to 59 mL/min) [[renal impairment]]. The safety of Moxidectin Tablets in patients with severe renal impairment (CrCL 15 to 29 mL/min) or end stage renal disease, is unknown. | |||
|useInHepaticImpair= | |||
|useInReproPotential= | |||
|useInImmunocomp= | |||
|othersTitle= | |||
|useInOthers= | |||
|administration= | |||
=====Recommended Dosage in Patients Aged 12 Years and Older===== | |||
*The recommended dosage of Moxidectin Tablet is a single dose of 8 mg (four 2 mg tablets) taken orally with or without food. | |||
|monitoring= | |||
|IVCompat= | |||
|overdose= | |||
*No specific antidote is available for [[overdose]] with Moxidectin Tablets. If overdose occurs, the patient should be monitored for evidence of toxicity. Treatment of overdose with Moxidectin Tablets consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient. Supportive therapy, if indicated, should include parenteral fluids and electrolytes, respiratory support (oxygen and mechanical ventilation if necessary) and pressor agents if clinically significant hypotension is present. | |||
|drugBox= | |||
{{drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 464194650 | |||
| IUPAC_name = ''(10E,14E,16E,22Z)-(1R,4S,5′S,6R,6′S,8R,13R,20R,21R,24S)''-6′- | |||
| image = MoxidectinStructure.png | |||
| alt = Structural formula of moxidectin | |||
| width = 250 | |||
| image2 = MoxidectinStructure3D.png | |||
| alt2 = Ball-and-stick model of the moxidectin molecule | |||
<!--Clinical data--> | |||
| tradename = Cydectin, Equest, ProHeart, Quest.<ref name="Awasthi 2013" /> | |||
| Drugs.com = {{drugs.com|international|moxidectin}} | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = | |||
| routes_of_administration = [[Oral administration|oral]], [[Topical medication|topical]], [[Subcutaneous injection|subcutaneous]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 113507-06-5 | |||
| CAS_supplemental = | |||
| ATCvet = yes | |||
| ATC_prefix = P54 | |||
| ATC_suffix = AB02 | |||
| ATC_supplemental = | |||
| PubChem = 9832912 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 16736424 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = NGU5H31YO9 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D05084 | |||
| synonyms = CL 301,423;<ref name="MesH">{{cite web|title=milbemycin|url=https://www.ncbi.nlm.nih.gov/mesh?cmd=Retrieve&LinkName=Full&list_uids=67027837|website=MeSH - NCBI|accessdate=2017-07-21}}</ref> milbemycin B.<ref name="MesH"/> | |||
== | <!--Chemical data--> | ||
* [ | | chemical_formula = | ||
* [ | | C=37 | H=53 | N=1 | O=8 | ||
* [ | | molecular_weight = 639.819 g/mol | ||
{{ | | smiles = CC(C)\C=C(/C)[C@H]5O[C@@]2(C[C@H]1OC(=O)[C@@H]3/C=C(/C)[C@@H](O)[C@H]4OC/C(=C\C=C\[C@H](C)CC(\C)=C\C[C@H](C1)O2)[C@@]34O)C\C(=N\OC)[C@@H]5C | ||
[[ | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChI = 1S/C37H53NO8/c1-21(2)14-25(6)33-26(7)31(38-42-8)19-36(46-33)18-29-17-28(45-36)13-12-23(4)15-22(3)10-9-11-27-20-43-34-32(39)24(5)16-30(35(40)44-29)37(27,34)41/h9-12,14,16,21-22,26,28-30,32-34,39,41H,13,15,17-20H2,1-8H3/b10-9+,23-12+,25-14+,27-11+,38-31-/t22-,26-,28+,29-,30-,32+,33+,34+,36-,37+/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = YZBLFMPOMVTDJY-CBYMMZEQSA-N | |||
}} | |||
|mechAction= | |||
*Moxidectin, a macrocyclic lactone, is an [[anthelmintic]] drug. | |||
|structure= | |||
*The empirical formula is C<sub>37</sub>H<sub>53</sub>NO<sub>8</sub> and the molecular weight is 639.82 Dalton. The structural formula is: | |||
[[File:MoxidectinStructure.png|600px|thumbnail|left|This image is provided by Wikipedia.]] | |||
{{clear}} | |||
|PD= | |||
Cardiac Electrophysiology | |||
*At a dose 4.5 times the approved recommended dose, moxidectin does not prolong the QT interval to any clinically relevant extent. | |||
|PK= | |||
*The pharmacokinetic parameters of moxidectin following a single 8 mg oral dose of Moxidectin Tablets to healthy subjects and patients with [[onchocerciasis]] under fasted conditions are shown in Table 4. Mean moxidectin Cmax and AUC increased approximately proportionally to dose over a dose range of 2 to 36 mg (0.25 to 4.5 times the approved recommended dose) in healthy subjects under fasted conditions. | |||
[[File:MoxidectinTable4.png|600px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clear}} | |||
Absorption<br> | |||
Effect of Food | |||
*Moxidectin mean Cmax and AUC increased on average by 34% and 39%, respectively, when administered with a standard high fat meal (900 calories, with a nutritional distribution of approximately 55% fat, 31% carbohydrates and 14% protein), compared to fasted conditions. | |||
Distribution | |||
*The apparent mean ± SD volume of distribution of moxidectin is 2421 ± 1658 L in patients with [[onchocerciasis]]. The plasma protein binding in humans is unknown. | |||
Elimination | |||
*The mean terminal half-life of moxidectin in patients with onchocerciasis is 23.3 days (559 hours) following a single 8 mg dose of Moxidectin Tablets. | |||
*The apparent mean ± SD total clearance of moxidectin is approximately 3.50 ± 1.23 L/hour in patients with [[onchocerciasis]]. | |||
Metabolism | |||
*The hepatic metabolism of moxidectin is minimal. | |||
Excretion | |||
*Following administration of a single 8 mg oral dose of Moxidectin Tablets to healthy subjects, 2% of the dose is eliminated unchanged in the feces within the first 72 hours. Renal elimination of intact drug is negligible. | |||
Specific Populations | |||
*In clinical studies, no clinically significant differences in the pharmacokinetics of moxidectin were observed based on age (18 to 60 years), sex, weight (42.7 to 107.2 kg), or renal impairment (creatinine clearance (CrCL) 47 to 89 mL/min, estimated by Cockcroft-Gault). The pharmacokinetics of moxidectin in patients with CrCL less than 47 mL/min is unknown. The pharmacokinetics of moxidectin in patients with [[hepatic impairment]] is unknown. | |||
Patients with Renal Impairment | |||
*Based on a population pharmacokinetic analysis and the fact that renal elimination of intact drug is negligible, mild (creatinine clearance (CrCL), estimated by Cockcroft-Gault of 60 to 89 mL/min) and moderate (CrCL 30 to 59 mL/min) renal impairment is not likely to have an impact on the exposure of moxidectin. The effect of severe renal impairment (CrCL 15 to 29 mL/min) or of end-stage renal disease on the pharmacokinetics of moxidectin is unknown. | |||
Drug Interaction Studies<br> | |||
Clinical Study with Midazolam (CYP3A4 substrate) | |||
*Co-administration of a single 8 mg dose of Moxidectin Tablets with a single oral 7.5 mg dose of midazolam (a sensitive CYP3A substrate) to healthy subjects (n = 37) did not affect the pharmacokinetics of midazolam or its major metabolite, 1-hydroxy midazolam. | |||
In Vitro Studies | |||
*CYP Enzymes: Moxidectin is not a substrate or inhibitor of CYP enzymes. | |||
*Uridine 5'-diphospho-glucuronosyltransferases (UGTs): Moxidectin is not a UGT substrate. | |||
*Transporter Systems: Moxidectin is not a substrate of P-glycoprotein (P-gp) nor breast cancer resistance protein 1 (BCRP1). | |||
===Microbiology=== | |||
Mechanism of Action | |||
*The mechanism by which moxidectin exhibits its effect against O. volvulus is not known. Studies with other nematodes suggest that moxidectin binds to glutamate-gated chloride channels (GluCl), gamma-aminobutyric acid (GABA) receptors and/or ATP-binding cassette (ABC) transporters. This leads to increased permeability, influx of chloride ions, hyperpolarization and muscle paralysis. Additionally, there is a reduction in motility of all stages of the parasite, excretion of immunomodulatory proteins, and the fertility of both male and female adult worms. | |||
Antimicrobial Activity | |||
*Moxidectin is active against the microfilariae of O. volvulus. | |||
*Studies suggest that moxidectin is not effective in killing the adult worms, however, it inhibits intra-uterine embryogenesis and release of microfilariae from the adult worms. | |||
Resistance | |||
*Studies in vitro and infected animals suggest a potential for development of resistance to moxidectin and cross-resistance with other macrocyclic lactones, such as ivermectin. However, the clinical relevance of these findings is not known. | |||
*The mechanism of resistance may be multifactorial that include alteration in the target GluCl, GABA receptors and/or ABC transporters. | |||
|nonClinToxic= | |||
=====Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |||
*Moxidectin was shown to be negative for genotoxicity in a battery of in vitro assays including a bacterial mutagenicity assay, mouse lymphoma cell mutagenicity assay, unscheduled DNA synthesis assay, and a chromosome aberration assay, as well as in vivo in a micronucleus assay in mice and a chromosome aberration assay in rats. | |||
*Two-year carcinogenicity studies in mice and rats were conducted with moxidectin. Mice were administered a mean dietary dose of 8.7 mg/kg/day moxidectin which is approximately equivalent to 5 times the recommended human dose based on body surface area comparison. Rats were administered a mean dietary dose of 6.1 mg/kg/day moxidectin which is approximately equivalent to 7 times the recommended human dose based on body surface area comparison. There was no evidence of tumorigenicity in either study. | |||
*In [[fertility]] evaluations, male and female mating and fertility indices were not inhibited by oral-dietary moxidectin doses of approximately 0.86 mg/kg/day which is approximately equivalent to the recommended human dose based on body surface area comparison. | |||
=====Animal Toxicology and/or Pharmacology===== | |||
*Moxidectin was associated with transient CNS-related clinical signs. In rats, a single dose of 20 mg/kg (equivalent to approximately 24 times the recommended human dose based on body surface area comparison) moxidectin was associated with piloerection, reduced arousal and body tone, abnormal gait, slowed breathing, and impaired righting reflex. In dogs, repeated doses of 1.6 mg/kg/day moxidectin (equivalent to approximately 7 times the recommended human dose based on body surface area comparison) was associated with [[lacrimation]], languid appearance, [[tremors]], slight salivation, and slight [[ataxia]]. | |||
|clinicalStudies= | |||
*The assessment of the safety and efficacy of Moxidectin Tablets 8 mg in the treatment of [[onchocerciasis]] is based on data from two randomized, double-blind, active-controlled trials in patients with O. volvulus infection, Trial 1 in 1472 patients (NCT 00790998), and Trial 2, a dose-ranging trial (NCT 00300768). Patients in the trials received a single oral dose of moxidectin or [[ivermectin]], the active control medication. | |||
*Efficacy was assessed by skin microfilarial density (microfilariae/mg skin) from the mean of 4 skin snips per person per time point up to 18 months post-treatment. | |||
*Trial 1 recruited adult and adolescent patients ≥ 12 years with a body weight ≥ 30 kg and ≥ 10 microfilariae/mg skin. Mean (± SD) age was 42.5 (± 16.3) years, height 1.59 (± 0.09) meters, weight 51.6 (± 8.2) kg; 36.1% were female and 100% were black. Mean (± SD) pretreatment skin microfilarial density was 39.5 (± 30.7), 69.6% had ≥ 20 microfilariae/mg skin and 39.7% had at least one ocular microfilaria. | |||
*Patients who were not previously exposed to ivermectin community directed treatment programs were recruited from the sub-Saharan African region (Democratic Republic of Congo, Liberia, and Ghana). Table 5 reports mean skin microfilarial density and the proportion of patients with undetectable skin microfilariae at Months 1, 6, and 12. | |||
[[File:MoxidectinTable5.png|600px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clear}} | |||
*Additionally, safety and efficacy was assessed in a smaller single ascending dose trial (Trial 2, NCT 00300768) comparing 2 mg (n = 44), 4 mg (n = 45) (2 mg and 4 mg are not approved doses) and 8 mg (n = 38) single doses of moxidectin to [[ivermectin]]. Trial 2 was conducted in Ghana in adults aged ≥ 18 to ≤ 60 years with O. volvulus infection. Analysis of the baseline-to-12-month change in skin microfilarial density for the proposed moxidectin 8 mg dose showed statistically significant superiority to [[ivermectin]], p < 0.001. | |||
|howSupplied= | |||
*Moxidectin Tablets containing 2 mg moxidectin are white to pale yellow uncoated oval-shaped tablets, debossed on one side with “AKKA”. Each high-density polyethylene bottle contains 500 tablets | |||
(NDC 71705-050-01), a silica gel desiccant and polyester coil. | |||
|NDC= | |||
|storage= | |||
*Store below 30°C (86°F). | |||
**Protect from light. | |||
**Once open, the full contents of the container should be used within 24 hours with any unused content discarded. | |||
|manBy= | |||
|distBy= | |||
|drugImages= | |||
|packLabel= | |||
|fdaPatientInfo= | |||
Signs and Symptoms Associated with Microfilarial Death | |||
*Advise patients that they are likely to have flu like symptoms including [[malaise]], [[myalgia]], [[headache]], [[tachycardia]], [[hypotension]] and [[pruritus]], most commonly during the first week after treatment. | |||
Symptomatic Orthostatic Hypotension | |||
*Advise patients that if they feel dizzy, faint or light-headed after taking Moxidectin Tablets, they should lie down until the symptoms resolve. | |||
Absence of Macrofilarial Activity | |||
*Advise patients that treatment with Moxidectin Tablets does not kill adult O. volvulus and that follow up evaluation is usually required. | |||
Edema and Worsening of Onchodermatitis | |||
*Advise patients with hyper-reactive onchodermatitis that they may be more likely to experience severe adverse reactions. | |||
Encephalopathy in Loa loa Co-infected Patients | |||
*Advise patients to report any symptoms of [[encephalopathy]] to their healthcare provider. | |||
|nlmPatientInfo= | |||
|alcohol= | |||
|brandNames=Moxidectin | |||
|lookAlike= | |||
|drugShortage= | |||
|price= | |||
}} | |||
Latest revision as of 16:28, 2 July 2019
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Moxidectin is an anthelmintic that is FDA approved for the treatment of onchocerciasis due to Onchocerca volvulus in patients aged 12 years and older. Common adverse reactions include eosinophilia, pruritus, musculoskeletal pain, headache, lymphopenia, tachycardia, rash, abdominal pain, hypotension, pyrexia, leukocytosis, influenza-like illness, neutropenia, cough, lymph node pain, dizziness, diarrhea, hyponatremia and peripheral swelling.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Moxidectin Tablets are indicated for the treatment of onchocerciasis due to Onchocerca volvulus in patients aged 12 years and older.
Limitations of Use
- Moxidectin Tablets do not kill adult O. volvulus. Follow-up evaluation is advised.
- The safety and efficacy of repeat administration of Moxidectin Tablets in patients with O. volvulus has not been studied.
Dosage
- The recommended dosage of Moxidectin Tablet is a single dose of 8 mg (four 2 mg tablets) taken orally with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding moxidectin Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding moxidectin Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indication
- Moxidectin Tablets are indicated for the treatment of onchocerciasis due to Onchocerca volvulus in patients aged 12 years and older.
- The safety and effectiveness of Moxidectin Tablets in pediatric patients under 12 years of age has not been established.
Limitations of Use
- Moxidectin Tablets do not kill adult O. volvulus. Follow-up evaluation is advised.
- The safety and efficacy of repeat administration of Moxidectin Tablets in patients with O. volvulus has not been studied.
Dosage
- The recommended dosage of Moxidectin Tablet for pediatric patients 12 years and older is a single dose of 8 mg (four 2 mg tablets) taken orally with or without food.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding moxidectin Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding moxidectin Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
Cutaneous, Ophthalmological and/or Systemic Adverse Reactions
- Treatment with Moxidectin Tablets may cause cutaneous, ophthalmological and/or systemic reactions of varying severity (Mazzotti reaction). These adverse reactions are due to allergic and inflammatory host responses to the death of microfilariae. There is a trend toward an increased incidence of these adverse reactions in patients with higher microfilarial burden.
- The clinical manifestations of Mazzotti reaction includes pruritus, headache, pyrexia, rash, urticaria, hypotension (including symptomatic orthostatic hypotension and dizziness), tachycardia, edema, lymphadenopathy, arthralgia, myalgia, chills, paresthesia and asthenia. Ophthalmological manifestations include conjunctivitis, eye pain, eye pruritus, eyelid swelling, blurred vision, photophobia, changes in visual acuity, hyperemia, ocular discomfort and watery eyes. These adverse reactions generally occur and resolve in the first week post-treatment. Laboratory changes include eosinophilia, eosinopenia, lymphocytopenia, neutropenia, and increases in alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT) and lactate dehydrogenase (LDH). Proteinuria has also been reported.
- Treatment of severe Mazzotti reactions has not been evaluated in controlled clinical trials. Symptomatic treatments such as oral hydration, recumbency, intravenous normal saline, and/or parenteral corticosteroids have been used to treat orthostatic hypotension. Antihistamines and/or analgesics have been used for most mild to moderate cases.
Symptomatic Orthostatic Hypotension
- An increased number of patients who received Moxidectin Tablets developed symptomatic orthostatic hypotension with inability to stand without support after lying down for 5 minutes (in an orthostatic hypotension provocation test); 47/978 (5%) compared with 8/494 (2%) who received ivermectin. The decreases in blood pressure were transient, managed by resumption of recumbency and most commonly occurred on Days 1 and 2 post-treatment. Advise patients that if they feel dizzy or light-headed after taking Moxidectin Tablets, they should lie down until the symptoms resolve.
Encephalopathy in Loa loa Co-infected Patients
- Patients with onchocerciasis who are also infected with Loa loa may develop a serious or even fatal
encephalopathy following treatment with Moxidectin Tablets.
- Moxidectin Tablets have not been studied in patients co-infected with Loa loa. Therefore, it is recommended that individuals who warrant treatment with Moxidectin Tablets and have had exposure to Loa loa-endemic areas undergo diagnostic screening for loiasis prior to treatment.
Edema and Worsening of Onchodermatitis
- Patients with hyper-reactive onchodermatitis (sowda) may be more likely than others to experience severe edema and worsening of onchodermatitis following the use of Moxidectin Tablets. Symptomatic treatment has been used to manage patients who have experienced edema and worsening of onchodermatitis.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under varying controlled conditions, adverse reaction rates observed in one clinical trial cannot be directly compared to rates observed in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The safety of Moxidectin Tablets was evaluated in two randomized, double-blind, active-controlled studies (Trial 1 and Trial 2) [see Clinical Studies]. In Trial 1, 978 patients received Moxidectin Tablets as a single oral dose of 8 mg and 494 patients received ivermectin as a single oral dose of approximately 150 mcg/kg. In Trial 2, 127 patients received Moxidectin Tablets as a single oral dose ranging from 2 mg (this is not an approved dose) to 8 mg (38 received the recommended 8 mg dose) and 45 patients received ivermectin as a single oral dose of approximately 150 mcg/kg.
Most Common Adverse Reactions
- No patients withdrew from either trial due to adverse reactions. Adverse Reactions reported in Trial 1 in > 10% of patients are summarized in Table 1. Most were related to physical, vital signs and laboratory changes associated with Mazzotti reaction.


- The most common adverse reactions in patients (n = 38) treated with 8 mg moxidectin in Trial 2 were similar to the adverse reactions noted in Trial 1 described in Table 1 above.
Other Adverse Reactions Reported in Clinical Trials
- The following adverse reactions occurred in less than 10% of subjects receiving Moxidectin Tablets in Trial 1:
- Ocular Adverse Reactions: In Trial 1, the most common ocular adverse reactions (occurring in ≥ 0.5% of patients) are shown in Table 2.

Hepatobiliary Adverse Reactions
- More patients in the moxidectin arm experienced elevation in bilirubin above the upper limit of normal and elevation in transaminases > 5x upper limit of normal. Twenty-seven (2.8%) patients in the moxidectin arm and 3 (0.6%) patients in the ivermectin arm had hyperbilirubinemia. Most of the patients had single measurements of hyperbilirubinemia without concurrent elevation in transaminases.
- Nine (1%) patients in the moxidectin arm and 2 (0.4%) patients in the ivermectin arm had elevation in ALT of more than 5x upper limit of normal; ten (1%) patients in the moxidectin arm and 3 (0.6%) patients in the ivermectin arm had elevation in AST to more than 5x upper limit of normal.
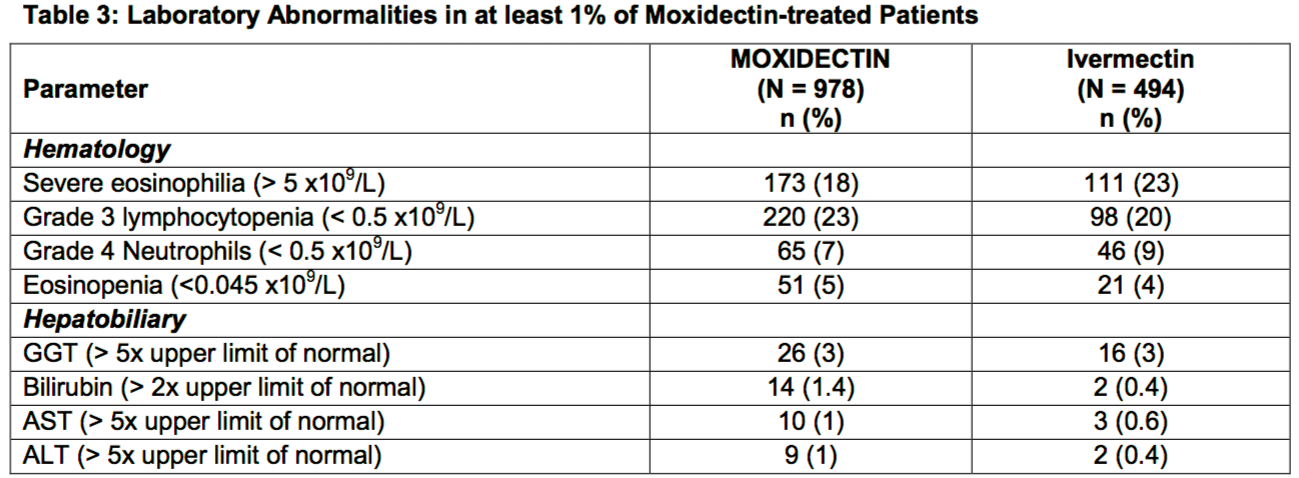
Laboratory Abnormalities
- Laboratory abnormalities occurring in at least 1% of patients in the Trial 1 are described in Table 3.
Postmarketing Experience
There is limited information regarding Moxidectin Postmarketing Experience in the drug label.
Drug Interactions
Midazolam (CYP3A4 substrate)
- In healthy subjects, concomitant administration of a single 8 mg oral dose of Moxidectin Tablets did not have an effect on the pharmacokinetics of midazolam. Moxidectin can be co-administered with CYP3A4 substrates.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- Limited available data on the use of Moxidectin Tablets in pregnant women are insufficient to establish whether there is a moxidectin-associated risk for major birth defects and miscarriage. Moxidectin administered orally to pregnant rats during the period of organogenesis (Gestation Days (GD) 6 to 15), was not associated with significant embryo-fetal developmental effects at doses of approximately 15 times the recommended human dose based on body surface area. When moxidectin was dosed orally to pregnant rabbits during the period of organogenesis (GD 7 - 19), no embryo-fetal developmental effects were observed at oral doses of moxidectin up to 24 times the recommended human dose based on body surface area.
- Daily parental oral administration of dietary moxidectin to rats prior to mating, and through mating, gestation, and lactation was associated with decreased survival and body weights for first-generation offspring without maternal toxicity at moxidectin doses less than 2-times the recommended human dose based on body surface area comparison. Daily dietary moxidectin did not produce maternal toxicity or adverse effects for first- and second-generation offspring at doses approximately equivalent to the recommended human dose based on body surface area comparison. Offspring were assessed for survival, body weights, and fertility. Developmental milestones were not assessed in this study.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
- In a rat embryo-fetal development study, daily oral administration of moxidectin at 12 mg/kg/day (approximately 15 times the recommended human dose of 8 mg based on body surface area comparison) during Gestation Days (GDs) 6 to 15 significantly increased the fetal incidence, but not the litter incidence of cleft palate and the fetal and litter incidence of a skeletal variation, wavy ribs, at a maternally toxic dose. Mean maternal food consumption, body weights, and body weight gain were significantly decreased at moxidectin doses of 10 and 12 mg/kg/day compared to control values. The no observed adverse effect level (NOAEL) value for maternal and fetal toxicity was considered to be 5 and 10 mg/kg/day respectively (approximately 6 and 12 times, respectively, the recommended human dose based on body surface area comparison). In the rabbit, daily oral administration of moxidectin at ≥ 5 mg/kg/day from GD7 to GD19 was not associated with fetal weight loss or malformations but resulted in significantly decreased maternal food consumption and body weight gains. The NOAEL value for maternal and fetal toxicity in the rabbit was 1 mg/kg/day and 10 mg/kg/day respectively (approximately 2 times and 24 times, respectively, the recommended human dose based on body surface area comparison). In a pre-postnatal study in rats, parental oral administration of dietary moxidectin prior to mating, through mating, gestation, and lactation did not produce adverse effects in first-generation or second- generation offspring at a maternal NOAEL dose of 0.824 mg/kg/day (approximately equivalent to the recommended human dose based on body surface area comparison). However, at moxidectin doses
≥ 1.1 mg/kg/day (approximately equivalent to 1.3 times the recommended human dose based on body surface area comparison), the survival and body weights of first-generation offspring were significantly decreased during the lactation period, and the number of live fetuses at birth was significantly decreased with a maternal moxidectin dose of 11 mg/kg/day (approximately equivalent to 13 times the recommended human dose based on body surface area comparison). In this study, offspring were assessed for survival, body weights, and fertility, and developmental milestones were not assessed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Moxidectin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Moxidectin during labor and delivery.
Nursing Mothers
Risk Summary
- Moxidectin was detected in the milk of lactating women following a single 8 mg dose of Moxidectin Tablets [see Data]. There are no data on the effects of Moxidectin Tablets on the breast-fed infant or milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Moxidectin Tablets and any potential adverse effects on the breastfed child from Moxidectin Tablets or from the underlying maternal condition.
Data
- A pharmacokinetic study in twelve healthy adult lactating women who were 21 to 100 weeks post partum evaluated the concentrations of moxidectin in plasma and breast milk collected over a period of 28 days following a single 8 mg dose of Moxidectin Tablets. The mean (± SD) exposure ratio of moxidectin present in human breast milk to that of human plasma was approximately 1.77 (± 0.66) over a collection period of
28 days. The estimated mean (± SD) total infant dose, assuming the infants would consume all the breast milk collected during the study, was 0.056 mg (± 0.024 mg), which would be approximately 0.70% (± 0.30%) of the maternal dose. The effects of moxidectin or its metabolites on the breast-fed child or milk production were not evaluated.
Pediatric Use
- The safety and effectiveness of Moxidectin Tablets have been established in pediatric patients 12 years of age and older. In Trial 1, (n = 53 patients aged 12 to 17 years), the safety and effectiveness was similar to that observed in adults. The safety and effectiveness of Moxidectin Tablets in pediatric patients under 12 years of age has not been established.
Geriatic Use
- Of the total number of patients included in Trial 1 that were treated with Moxidectin Tablets, 83 were aged 65 and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Moxidectin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Moxidectin with respect to specific racial populations.
Renal Impairment
- No dose adjustment of Moxidectin Tablets is necessary for patients with mild (creatinine clearance (CrCL) 60 to 89 mL/min) to moderate (CrCL 30 to 59 mL/min) renal impairment. The safety of Moxidectin Tablets in patients with severe renal impairment (CrCL 15 to 29 mL/min) or end stage renal disease, is unknown.
Hepatic Impairment
There is no FDA guidance on the use of Moxidectin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Moxidectin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Moxidectin in patients who are immunocompromised.
Administration and Monitoring
Administration
Recommended Dosage in Patients Aged 12 Years and Older
- The recommended dosage of Moxidectin Tablet is a single dose of 8 mg (four 2 mg tablets) taken orally with or without food.
Monitoring
There is limited information regarding Moxidectin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Moxidectin and IV administrations.
Overdosage
- No specific antidote is available for overdose with Moxidectin Tablets. If overdose occurs, the patient should be monitored for evidence of toxicity. Treatment of overdose with Moxidectin Tablets consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient. Supportive therapy, if indicated, should include parenteral fluids and electrolytes, respiratory support (oxygen and mechanical ventilation if necessary) and pressor agents if clinically significant hypotension is present.
Pharmacology
| |
| |
Moxidectin
| |
| Systematic (IUPAC) name | |
| (10E,14E,16E,22Z)-(1R,4S,5′S,6R,6′S,8R,13R,20R,21R,24S)-6′- | |
| Identifiers | |
| CAS number | |
| ATC code | P54 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 639.819 g/mol |
| SMILES | & |
| Synonyms | CL 301,423;[1] milbemycin B.[1] |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | oral, topical, subcutaneous |
Mechanism of Action
- Moxidectin, a macrocyclic lactone, is an anthelmintic drug.
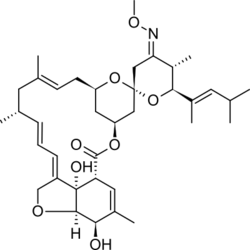
Structure
- The empirical formula is C37H53NO8 and the molecular weight is 639.82 Dalton. The structural formula is:

Pharmacodynamics
Cardiac Electrophysiology
- At a dose 4.5 times the approved recommended dose, moxidectin does not prolong the QT interval to any clinically relevant extent.
Pharmacokinetics
- The pharmacokinetic parameters of moxidectin following a single 8 mg oral dose of Moxidectin Tablets to healthy subjects and patients with onchocerciasis under fasted conditions are shown in Table 4. Mean moxidectin Cmax and AUC increased approximately proportionally to dose over a dose range of 2 to 36 mg (0.25 to 4.5 times the approved recommended dose) in healthy subjects under fasted conditions.

Absorption
Effect of Food
- Moxidectin mean Cmax and AUC increased on average by 34% and 39%, respectively, when administered with a standard high fat meal (900 calories, with a nutritional distribution of approximately 55% fat, 31% carbohydrates and 14% protein), compared to fasted conditions.
Distribution
- The apparent mean ± SD volume of distribution of moxidectin is 2421 ± 1658 L in patients with onchocerciasis. The plasma protein binding in humans is unknown.
Elimination
- The mean terminal half-life of moxidectin in patients with onchocerciasis is 23.3 days (559 hours) following a single 8 mg dose of Moxidectin Tablets.
- The apparent mean ± SD total clearance of moxidectin is approximately 3.50 ± 1.23 L/hour in patients with onchocerciasis.
Metabolism
- The hepatic metabolism of moxidectin is minimal.
Excretion
- Following administration of a single 8 mg oral dose of Moxidectin Tablets to healthy subjects, 2% of the dose is eliminated unchanged in the feces within the first 72 hours. Renal elimination of intact drug is negligible.
Specific Populations
- In clinical studies, no clinically significant differences in the pharmacokinetics of moxidectin were observed based on age (18 to 60 years), sex, weight (42.7 to 107.2 kg), or renal impairment (creatinine clearance (CrCL) 47 to 89 mL/min, estimated by Cockcroft-Gault). The pharmacokinetics of moxidectin in patients with CrCL less than 47 mL/min is unknown. The pharmacokinetics of moxidectin in patients with hepatic impairment is unknown.
Patients with Renal Impairment
- Based on a population pharmacokinetic analysis and the fact that renal elimination of intact drug is negligible, mild (creatinine clearance (CrCL), estimated by Cockcroft-Gault of 60 to 89 mL/min) and moderate (CrCL 30 to 59 mL/min) renal impairment is not likely to have an impact on the exposure of moxidectin. The effect of severe renal impairment (CrCL 15 to 29 mL/min) or of end-stage renal disease on the pharmacokinetics of moxidectin is unknown.
Drug Interaction Studies
Clinical Study with Midazolam (CYP3A4 substrate)
- Co-administration of a single 8 mg dose of Moxidectin Tablets with a single oral 7.5 mg dose of midazolam (a sensitive CYP3A substrate) to healthy subjects (n = 37) did not affect the pharmacokinetics of midazolam or its major metabolite, 1-hydroxy midazolam.
In Vitro Studies
- CYP Enzymes: Moxidectin is not a substrate or inhibitor of CYP enzymes.
- Uridine 5'-diphospho-glucuronosyltransferases (UGTs): Moxidectin is not a UGT substrate.
- Transporter Systems: Moxidectin is not a substrate of P-glycoprotein (P-gp) nor breast cancer resistance protein 1 (BCRP1).
Microbiology
Mechanism of Action
- The mechanism by which moxidectin exhibits its effect against O. volvulus is not known. Studies with other nematodes suggest that moxidectin binds to glutamate-gated chloride channels (GluCl), gamma-aminobutyric acid (GABA) receptors and/or ATP-binding cassette (ABC) transporters. This leads to increased permeability, influx of chloride ions, hyperpolarization and muscle paralysis. Additionally, there is a reduction in motility of all stages of the parasite, excretion of immunomodulatory proteins, and the fertility of both male and female adult worms.
Antimicrobial Activity
- Moxidectin is active against the microfilariae of O. volvulus.
- Studies suggest that moxidectin is not effective in killing the adult worms, however, it inhibits intra-uterine embryogenesis and release of microfilariae from the adult worms.
Resistance
- Studies in vitro and infected animals suggest a potential for development of resistance to moxidectin and cross-resistance with other macrocyclic lactones, such as ivermectin. However, the clinical relevance of these findings is not known.
- The mechanism of resistance may be multifactorial that include alteration in the target GluCl, GABA receptors and/or ABC transporters.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Moxidectin was shown to be negative for genotoxicity in a battery of in vitro assays including a bacterial mutagenicity assay, mouse lymphoma cell mutagenicity assay, unscheduled DNA synthesis assay, and a chromosome aberration assay, as well as in vivo in a micronucleus assay in mice and a chromosome aberration assay in rats.
- Two-year carcinogenicity studies in mice and rats were conducted with moxidectin. Mice were administered a mean dietary dose of 8.7 mg/kg/day moxidectin which is approximately equivalent to 5 times the recommended human dose based on body surface area comparison. Rats were administered a mean dietary dose of 6.1 mg/kg/day moxidectin which is approximately equivalent to 7 times the recommended human dose based on body surface area comparison. There was no evidence of tumorigenicity in either study.
- In fertility evaluations, male and female mating and fertility indices were not inhibited by oral-dietary moxidectin doses of approximately 0.86 mg/kg/day which is approximately equivalent to the recommended human dose based on body surface area comparison.
Animal Toxicology and/or Pharmacology
- Moxidectin was associated with transient CNS-related clinical signs. In rats, a single dose of 20 mg/kg (equivalent to approximately 24 times the recommended human dose based on body surface area comparison) moxidectin was associated with piloerection, reduced arousal and body tone, abnormal gait, slowed breathing, and impaired righting reflex. In dogs, repeated doses of 1.6 mg/kg/day moxidectin (equivalent to approximately 7 times the recommended human dose based on body surface area comparison) was associated with lacrimation, languid appearance, tremors, slight salivation, and slight ataxia.
Clinical Studies
- The assessment of the safety and efficacy of Moxidectin Tablets 8 mg in the treatment of onchocerciasis is based on data from two randomized, double-blind, active-controlled trials in patients with O. volvulus infection, Trial 1 in 1472 patients (NCT 00790998), and Trial 2, a dose-ranging trial (NCT 00300768). Patients in the trials received a single oral dose of moxidectin or ivermectin, the active control medication.
- Efficacy was assessed by skin microfilarial density (microfilariae/mg skin) from the mean of 4 skin snips per person per time point up to 18 months post-treatment.
- Trial 1 recruited adult and adolescent patients ≥ 12 years with a body weight ≥ 30 kg and ≥ 10 microfilariae/mg skin. Mean (± SD) age was 42.5 (± 16.3) years, height 1.59 (± 0.09) meters, weight 51.6 (± 8.2) kg; 36.1% were female and 100% were black. Mean (± SD) pretreatment skin microfilarial density was 39.5 (± 30.7), 69.6% had ≥ 20 microfilariae/mg skin and 39.7% had at least one ocular microfilaria.
- Patients who were not previously exposed to ivermectin community directed treatment programs were recruited from the sub-Saharan African region (Democratic Republic of Congo, Liberia, and Ghana). Table 5 reports mean skin microfilarial density and the proportion of patients with undetectable skin microfilariae at Months 1, 6, and 12.

- Additionally, safety and efficacy was assessed in a smaller single ascending dose trial (Trial 2, NCT 00300768) comparing 2 mg (n = 44), 4 mg (n = 45) (2 mg and 4 mg are not approved doses) and 8 mg (n = 38) single doses of moxidectin to ivermectin. Trial 2 was conducted in Ghana in adults aged ≥ 18 to ≤ 60 years with O. volvulus infection. Analysis of the baseline-to-12-month change in skin microfilarial density for the proposed moxidectin 8 mg dose showed statistically significant superiority to ivermectin, p < 0.001.
How Supplied
- Moxidectin Tablets containing 2 mg moxidectin are white to pale yellow uncoated oval-shaped tablets, debossed on one side with “AKKA”. Each high-density polyethylene bottle contains 500 tablets
(NDC 71705-050-01), a silica gel desiccant and polyester coil.
Storage
- Store below 30°C (86°F).
- Protect from light.
- Once open, the full contents of the container should be used within 24 hours with any unused content discarded.
Images
Drug Images
{{#ask: Page Name::Moxidectin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Moxidectin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Signs and Symptoms Associated with Microfilarial Death
- Advise patients that they are likely to have flu like symptoms including malaise, myalgia, headache, tachycardia, hypotension and pruritus, most commonly during the first week after treatment.
Symptomatic Orthostatic Hypotension
- Advise patients that if they feel dizzy, faint or light-headed after taking Moxidectin Tablets, they should lie down until the symptoms resolve.
Absence of Macrofilarial Activity
- Advise patients that treatment with Moxidectin Tablets does not kill adult O. volvulus and that follow up evaluation is usually required.
Edema and Worsening of Onchodermatitis
- Advise patients with hyper-reactive onchodermatitis that they may be more likely to experience severe adverse reactions.
Encephalopathy in Loa loa Co-infected Patients
- Advise patients to report any symptoms of encephalopathy to their healthcare provider.
Precautions with Alcohol
Alcohol-Moxidectin interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Moxidectin
Look-Alike Drug Names
There is limited information regarding Moxidectin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 "milbemycin". MeSH - NCBI. Retrieved 2017-07-21.