Leishmaniasis
Template:DiseaseDisorder infobox Template:Search infobox Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Leishmaniasis is a disease caused by protozoan parasites that belong to the genus Leishmania and is transmitted by the bite of certain species of sand fly, including flies in the genus Lutzomyia in the New World and Phlebotomus in the Old World. The disease was named in 1901 for the Scottish pathologist William Boog Leishman. This disease is also known as Leichmaniosis, Leishmaniose, leishmaniose, and formerly, Orient Boils, Baghdad Boil, kala azar, black fever, sandfly disease, Dum-Dum fever or espundia.
Most forms of the disease are transmissible only from animals (zoonosis), but some can be spread between humans. Human infection is caused by about 21 of 30 species that infect mammals. These include the L. donovani complex with three species (L. donovani, L. infantum, and L. chagasi); the L. mexicana complex with 3 main species (L. mexicana, L. amazonensis, and L. venezuelensis); L. tropica; L. major; L. aethiopica; and the subgenus Viannia with four main species (L. (V.) braziliensis, L. (V.) guyanensis, L. (V.) panamensis, and L. (V.) peruviana). The different species are morphologically indistinguishable, but they can be differentiated by isoenzyme analysis, DNA sequence analysis, or monoclonal antibodies.
Visceral leishmaniasis is a severe form in which the parasites have migrated to the vital organs.
Epidemiology and Demographics
The number of new cases of cutaneous leishmaniasis each year in the world is thought to be about 1.5 million. The number of new cases of visceral leishmaniasis is thought to be about 500,000.
Leishmaniasis is found in parts of about 88 countries. Approximately 350 million people live in these areas. Most of the affected countries are in the tropics and subtropics. The settings in which leishmaniasis is found range from rain forests in Central and South America to deserts in West Asia. More than 90 percent of the world's cases of visceral leishmaniasis are in India, Bangladesh, Nepal, Sudan, and Brazil.
Leishmaniasis is found in some parts of the following areas:
- in Mexico, Central America, and South America -- from northern Argentina to Texas (not in Uruguay, Chile, or Canada)
- southern Europe (leishmaniasis is not common in travelers to southern Europe)
- Asia (not Southeast Asia)
- the Middle East
- Africa (particularly East and North Africa, with some cases elsewhere)
Leishmaniasis is not found in Australia or Oceania (that is, islands in the Pacific, including Melanesia, Micronesia, and Polynesia).
Leishmaniasis can be transmitted in many tropical and sub-tropical countries, and is found in parts of about 88 countries. Approximately 350 million people live in these areas. The settings in which leishmaniasis is found range from rainforests in Central and South America to deserts in West Asia. More than 90 percent of the world's cases of visceral leishmaniasis are in India, Bangladesh, Nepal, Sudan, and Brazil.
Leishmaniasis is commonly found in Mexico, Central America, and South America—from northern Argentina to southern Texas (not in Uruguay, Chile, or Canada), southern Europe (leishmaniasis is not common in travelers to southern Europe), Asia (not Southeast Asia), the Middle East, and Africa (particularly East and North Africa, with some cases elsewhere). It has recently been shown to be spreading to North Texas [1]. The disease is not found in Australia or Oceania.
Leishmaniasis is present in Iraq and was contracted by a number of the troops involved in the 2003 invasion of that country and the subsequent occupation. The soldiers nicknamed the disease the Baghdad boil. It has been reported by Agence France-Presse that more than 650 U.S. soldiers may have experienced the disease between the start of the invasion in March 2003 and late 2004. [3] [4]
In fact, U.S. troops have experienced leishmaniasis cases in the Middle East already previously to the 2003 invasion, during the time of the previous Gulf conflict, when a large number of soldiers were stationed in Saudi Arabia. Of the 32 cases that were recorded by the U.S. military for that period (1990-1991), 20 were cutaneous, and 12 of the more severe visceral type [5].
During 2004, it is calculated that some 3,400 troops from the Colombian army, operating in the jungles near the south of the country (in particular around the Meta and Guaviare departments), were infected with Leishmaniasis. Apparently, a contributing factor was that many of the affected soldiers did not use the officially provided insect repellent, because of its allegedly disturbing odor. It is estimated that nearly 13,000 cases of the disease were recorded in all of Colombia throughout 2004, and about 360 new instances of the disease among soldiers had been reported in February 2005. [6] [7] [8]
In September 2005 the disease was contracted by at least four Dutch marines who were stationed in Mazari Sharif, Afghanistan, and subsequently repatriated for treatment.
Within Afghanistan, in particular Kabul is a town where leishmaniasis occurs commonly - partly to do with bad sanitation and waste left uncollected in streets, allowing parasite-spreading sand flies an environment they find favorable. See e.g. [9] and [10]. In Kabul the number of people infected is estimated at at least 200,000, and in three other towns (Herat, Kandahar and Mazar-i-Sharif) there may be about 70,000 more, according to WHO figures from 2002 cited e.g. here: [11].
Could I get leishmaniasis in the United States?
Probably not. It is possible but very unlikely that you would get leishmaniasis in the United States. Very rarely, people living in Texas have developed skin sores from cutaneous leishmaniasis.
No cases of visceral leishmaniasis are known to have been acquired in the United States.
Risk Factors
People of all ages are at risk for leishmaniasis if they live or travel where leishmaniasis is found. Leishmaniasis usually is more common in rural than urban areas; but it is found in the outskirts of some cities. The risk for leishmaniasis is highest from dusk to dawn because this is when sand flies are the most active. All it takes to get infected is to be bitten by one infected sand fly. This is more likely to happen the more people are bitten, that is, the more time they spend outside in rural areas from dusk to dawn. Adventure travelers, Peace Corps volunteers, missionaries, ornithologists (people who study birds), other people who do research outdoors at night, and soldiers are examples of people who may have an increased risk for leishmaniasis (especially cutaneous leishmaniasis).
Pathophysiology
Leishmaniasis is spread by the bite of some types of phlebotomine sand flies. Sand flies become infected by biting an infected animal (for example, a rodent or dog) or person. Since sand flies do not make noise when they fly, people may not realize they are present. Sand flies are very small and may be hard to see; they are only about one-third the size of typical mosquitoes. Sand flies usually are most active in twilight, evening, and night-time hours (from dusk to dawn). Sand flies are less active during the hottest time of the day. However, they will bite if they are disturbed, such as when a person brushes up against the trunk of a tree where sand flies are resting. Rarely, leishmaniasis is spread from a pregnant woman to her baby. Leishmaniasis also can be spread by blood transfusions or contaminated needles.
Life cycle
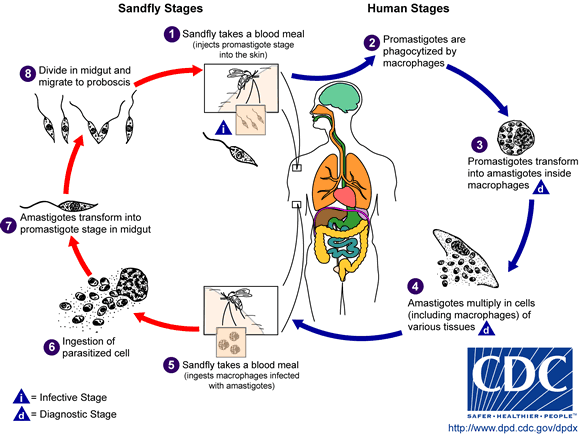
Leishmaniasis is transmitted by the bite of female phlebotomine sandflies. The sandflies inject the infective stage, metacyclic promastigotes, during blood meals (1). Metacyclic promastigotes that reach the puncture wound are phagocytized by macrophages (2) and transform into amastigotes (3). Amastigotes multiply in infected cells and affect different tissues, depending in part on which Leishmania species is involved (4). These differing tissue specificities cause the differing clinical manifestations of the various forms of leishmaniasis. Sandflies become infected during blood meals on an infected host when they ingest macrophages infected with amastigotes (5,6). In the sandfly's midgut, the parasites differentiate into promastigotes (7), which multiply, differentiate into metacyclic promastigotes and migrate to the proboscis (8)
Signs and symptoms
Human leishmanial infections can result in 2 main forms of disease, cutaneous leishmaniasis and visceral leishmaniasis (kala-azar). The factors determining the form of disease include leishmanial species, geographic location, and immune response of the host.
- Cutaneous leishmaniasis is characterized by one or more cutaneous lesions on areas where sandflies have fed. Persons who have cutaneous leishmaniasis have one or more sores on their skin. The sores can change in size and appearance over time. They often end up looking somewhat like a volcano, with a raised edge and central crater. A scab covers some sores. The sores can be painless or painful. Some people have swollen glands near the sores (for example, in the armpit if the sores are on the arm or hand).
- Persons who have visceral leishmaniasis usually have fever, weight loss, and an enlarged spleen and liver (usually the spleen is bigger than the liver). Some patients have swollen glands. Certain blood tests are abnormal. For example, patients usually have low blood counts, including a low red blood cell count (anemia), low white blood cell count, and low platelet count. Some patients develop post kala-azar dermal leishmaniasis. Visceral leishmaniasis is becoming an important opportunistic infection in areas where it coexists with HIV.
In the medical field, leishmaniasis is one of the famous causes of a markedly enlarged spleen, which may become larger even than the liver. There are four main forms of leishmaniasis:
- Visceral leishmaniasis - the most serious form and potentially fatal if untreated.
- Cutaneous leishmaniasis - the most common form which causes a sore at the bite site, which heal in a few months to a year, leaving an unpleasant looking scar. This form can progress to any of the other three forms.
- Diffuse cutaneous leishmaniasis - this form produces widespread skin lesions which resemble leprosy and is particularly difficult to treat.
- Mucocutaneous leishmaniasis - commences with skin ulcers which spread causing tissue damage to (particularly) nose and mouth
If I were bitten by an infected sand fly, how quickly would I become sick?
People with cutaneous leishmaniasis usually develop skin sores within a few weeks (sometimes as long as months) of when they were bitten.
People with visceral leishmaniasis usually become sick within several months (rarely as long as years) of when they were bitten. The symptoms of leishmaniasis are skin sores which erupt weeks to months after the person affected is bitten by sand flies. Other consequences, which can become manifest anywhere from a few months to years after infection, include fever, damage to the spleen and liver, and anaemia.
Treatment
There are two common therapies containing antimony (known as pentavalent antimonials), meglumine antimoniate (Glucantim®) and sodium stibogluconate (Pentostam®). It is not completely understood how these drugs act against the parasite; they may disrupt its energy production or trypanothione metabolism. Unfortunately, in many parts of the world, the parasite has become resistant to antimony and for visceral or mucocutaneous leishmaniasis,[2] but the level of resistance varies according to species.[3] Amphotericin is now the treatment of choice[4]; failure of AmBisome® to treat visceral leishmaniasis (Leishmania donovani) has been reported in Sudan,[5] but this failure may be related to host factors such as co-infection with HIV or tuberculosis rather than parasite resistance.
Miltefosine (Impavido®), is a new drug for visceral and cutaneous leishmaniasis. The cure rate of miltefosine in phase III clinical trials is 95%; Studies in Ethiopia show that is also effective in Africa. In HIV immunosuppressed people who are coinfected with leishmaniasis it has shown that even in resistant cases 2/3 of the people responded to this new treatment. Clinical trials in Colombia showed a high efficacy for cutaneous leishmaniasis. In mucocutaneous cases caused by L.brasiliensis it has shown to be more effective than other drugs. Miltefosine received approval by the Indian regulatory authorities in 2002 and in Germany in 2004. In 2005 it received the first approval for cutaneous leishmaniasis in Colombia. Miltefosine is also currently being investigated as treatment for mucocutaneous leishmaniasis caused by Leishmania braziliensis in Colombia,[2] and preliminary results are very promising. It is now registered in many countries and is the first orally administered breakthrough therapy for visceral and cutaneous leishmaniasis.[6](More, et al, 2003). In October 2006 it received orphan drug status from the US Food and Drug administration. The drug is generally better tolerated than other drugs. Main side effects are gastrointetinal disturbance in the 1-2 days of treatment which does not affect the efficacy. Because it is available as an oral formulation, the expense and inconvenience of hospitalisation is avoided, which makes it an attractive alternative.
The Institute for OneWorld Health has developed paromomycin, results with which led to its approval as an orphan drug. The Drugs for Neglected Diseases Initiative is also actively facilitating the search for novel therapeutics.
Drug-resistant leishmaniasis may respond to immunotherapy (inoculation with parasite antigens plus an adjuvant) which aims to stimulate the body's own immune system to kill the parasite.[7]
Several potential vaccines are being developed, under pressure from the World Health Organization, but as of 2006 none is available. The team at the Laboratory for Organic Chemistry at the Swiss Federal Institute of Technology (ETH) in Zürich are trying to design a carbohydrate-based vaccine [12]. The genome of the parasite Leishmania major has been sequenced,[8] possibly allowing for identification of proteins that are used by the pathogen but not by humans; these proteins are potential targets for drug treatments.
History
Description of conspicuous lesions similar to cutaneous Leishmaniasis (CL) has been discovered on tablets from King Ashurbanipal from the 7th century BCE, some of which may have been derived from even earlier texts from 1500 to 2500 BCE. Arab physicians including Avicenna in the 10th century gave detailed description of what was called Balkh sore[9]. In 1756, Alexander Russell, after examining a Turkish patient, gave one of the most detailed clinical description of the disease. Physicians in the Indian Subcontinent would describe it as Kala-azar (pronounced kālā āzār, the Urdu, Hindi and Hindustani phrase for black fever, kālā meaning black and āzār meaning fever or disease). As for the new world, evidence of cutaneous form of the disease was found in Ecuador and Peru in pre-Inca potteries depicting skin lesions and deformed faces dating back to the first century CE. 15th and 16th century texts from Inca period and from spanish colonials mention "valley sickness", "Andean sickness" or "white leprosy" which are likely to be CL[10].
Who first discovered the organism is somewhat disputed. It is possible that Surgeon major David D Cunnigham of British Indian army saw it first in 1885 without being able to relate it to the disease[11][12]. In 1901,William Boog Leishman identified certain organisms in smears taken from the spleen of a patient who had died from "dum-dum fever" (Dhum dhum is an area close to Calcutta) and in 1903 Captain Charles Donovan (1863-1951) described them as being new organism[10]. Eventually Ronald Ross established the link with the disease and named the organism Leishmania donovani.
Leishmaniasis as part of the CVBDs
CVBD stands for Canine Vector-borne diseases, which are diseases transmitted through Ectoparasites.
Diagnosis
Examination of Giemsa stained slides of the relevant tissue is still the technique most commonly used to detect the parasite.
Laboratory Findings
Microscopy:
Isolation of the organism in culture (using for example the diphasic NNN medium) or in experimental animals (hamsters) constitutes another method of parasitilogic confirmation of the diagnosis, and in addition can provide material for further investigations (e.g., isoenzyme analysis). Antibody detection can prove useful in visceral leishmaniasis but is of limited value in cutaneous disease, where most patients do not develop a significant antibody response. In addition, cross reactivity can occur with Trypanosoma cruzi, a fact to consider when investigating Leishmania antibody response in patients who have been in Central or South America. Other diagnostic techniques exist that allow parasite detection and/or species identification using biochemical (isoenzymes), immunologic (immunoassays), and molecular (PCR) approaches. Such techniques, however, are not readily available in general diagnostic laboratories.
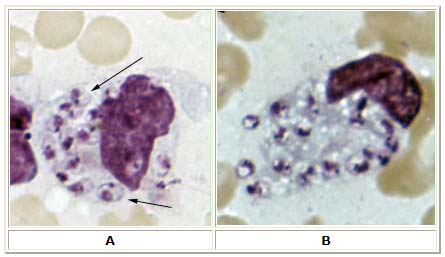
A, B: Leishmania tropica amastigotes from an impression smear of a biopsy specimen from a skin lesion. In A, an intact macrophage is practically filled with amastigotes (arrows), several of which have a clearly visible nucleus and kinetoplast; in B, amastigotes are being freed from a rupturing macrophage. Patient had traveled to Egypt, Africa, and the Middle East. Based on culture in NNN medium, followed by isoenzyme analysis, the species was L. tropica.
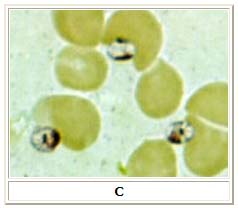
C: Three Leishmania amastigotes, each with a clearly visible nucleus and kinetoplast, from the same impression smear as in A and B.
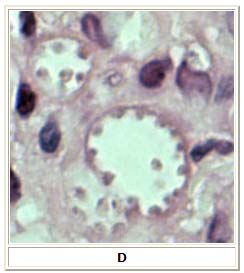
D: Leishmania mexicana in a biopsy specimen from a skin lesion stained with hematoxylin and eosin. The amastigotes are lining the walls of two vacuoles, a typical arrangement. The species identification was derived from culture followed by isoenzyme analysis. Infection was acquired in Texas.
Risk Stratification and Prognosis
The skin sores of cutaneous leishmaniasis will heal on their own, but this can take months or even years. The sores can leave ugly scars. If not treated, infection that started in the skin rarely spreads to the nose or mouth and causes sores there (mucosal leishmaniasis). This can happen with some of the types of the parasite found in Central and South America. Mucosal leishmaniasis might not be noticed until years after the original skin sores healed. The best way to prevent mucosal leishmaniasis is to treat the cutaneous infection before it spreads.
- If not treated, visceral leishmaniasis can cause death.
- Some people have had cutaneous leishmaniasis more than once.
Treatment
Pharmacotherapy
Acute Pharmacotherapies
Physicians may consult CDC to obtain information on how to treat leishmaniasis. The drug sodium stibogluconate is available under an Investigational New Drug protocol from the CDC Drug Service.
Chronic Pharmacotherapies
Primary Prevention
The best way for travelers to prevent leishmaniasis is by protecting themselves from sand fly bites. Vaccines and drugs for preventing infection are not yet available. To decrease their risk of being bitten, travelers should:
- Stay in well-screened or air-conditioned areas as much as possible. Avoid outdoor activities, especially from dusk to dawn, when sand flies are the most active.
- When outside, wear long-sleeved shirts, long pants, and socks. Tuck your shirt into your pants.
- Apply insect repellent on uncovered skin and under the ends of sleeves and pant legs. Follow the instructions on the label of the repellent. The most effective repellents are those that contain the chemical DEET (N,N-diethylmetatoluamide). The concentration of DEET varies among repellents. Repellents with DEET concentrations of 30-35% are quite effective, and the effect should last about 4 hours. Lower concentrations should be used for children (no more than 10% DEET). Repellents with DEET should be used sparingly on children from 2 to 6 years old and not at all on children less than 2 years old.
- Spray clothing with permethrin-containing insecticides. The insecticide should be reapplied after every five washings.
- Spray living and sleeping areas with an insecticide to kill insects.
- If you are not sleeping in an area that is well screened or air-conditioned, use a bed net and tuck it under your mattress. If possible, use a bed net that has been soaked in or sprayed with permethrin. The permethrin will be effective for several months if the bed net is not washed. Keep in mind that sand flies are much smaller than mosquitoes and therefore can get through smaller holes. Fine-mesh netting (at least 18 holes to the inch; some sources say even finer) is needed for an effective barrier against sand flies. This is particularly important if the bed net has not been treated with permethrin. However, it may be uncomfortable to sleep under such a closely woven bed net when it is hot.
NOTE: Bed nets, repellents containing DEET, and permethrin should be purchased before traveling and can be found in hardware, camping, and military surplus stores.
See also
- Visceral leishmaniasis (kala azar)
- Cutaneous leishmaniasis
- Leishmania
References
- ↑ "Houston Chronicle: Texas Doctors Find Skin Disease Moving North". Retrieved 2007-09-15.
- ↑ 2.0 2.1 Soto J, Toledo JT. "Oral miltefosine to treat new world cutaneous leishmaniasis". Lancet Infect Dis. 7 (1): 7.
- ↑ Arevalo J, Ramirez L, Adaui V; et al. (2007). "Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis". J Infect Dis. 195: 1846&ndash, 51. doi:10.1086/518041.
- ↑ Sundar S, Chakravarty J, Rai VK; et al. (2007). "Amphotericin B Treatment for Indian Visceral Leishmaniasis: Response to 15 Daily versus Alternate-Day Infusions". Clin Infect Dis. 45: 556&ndash, 561.
- ↑ Mueller M, Ritmeijer K, Balasegaram M, Koummuki Y, Santana MR, Davidson R. (2007). "Unresponsiveness to AmBisome® in some Sudanese patients with kala-azar". Trans R Soc Trop Med Hyg. 101 (1): 19&ndash, 24. doi:10.1016/j.trstmh.2006.02.005.
- ↑ Jha TK, Sundar S, Thakur CP; et al. (1999). "Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis". New Engl J Med. 341: 1795&ndash, 800.
- ↑ Badaro R, Lobo I, Munõs A; et al. (2006). "Immunotherapy for drug-refractory mucosal leishmaniasis". J Infect Dis. 194: 1151&ndash, 59.
- ↑ Ivens AC; et al. (2005). "The genome of the kinetoplastid parasite, Leishmania major". Science. 309 (5733): 436&ndash, 42. PMID 16020728.
- ↑ Cox, Francis E G (1996). The Wellcome Trust illustrated history of tropical diseases. London: The Wellcome Trust. pp. 206–217. ISBN 1869835867, 9781869835866 Check
|isbn=value: invalid character (help). OCLC 35161690. - ↑ 10.0 10.1 "WHO: Leishmaniasis: background information". Retrieved 2007-07-04.
- ↑ Cunningham, DD (1885). On the presence of peculiar parasitic organisms in the tissue of a specimen of Delhi boil. Scientific memoirs officers Medical Sanitary Departments Government India. Calcutta: Printed by the superintendent of government printing, India. pp. 21–31. OCLC 11826455.
- ↑ Cox FE (2002). "History of human parasitology". Clin. Microbiol. Rev. 15 (4): 595–612. PMID 12364371.
External links
- Doctors Without Borders' leishmaniasis information page
- Drugs for Neglected Diseases Initiative
- International Leishmania Network
- Leish-L discussion list
- Wanted: social entrepreneurs Nature 434, 941 (21 April 2005)
- "Symposium issue". Journal of Postgraduate Medicine. 49 (1). 2003.
- Open Directory Project - Leishmaniasis directory category
ar:كالازار ca:Leishmaniosi de:Leishmaniose it:Leishmaniosi umana he:שושנת יריחו nl:Leishmaniasis qu:Uta sv:Sandmyggefeber