Lamivudine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 124: | Line 124: | ||
======Clinical Trials in HIV-1====== | ======Clinical Trials in HIV-1====== | ||
Limited short-term safety information is available from 2 small, uncontrolled trials in South Africa in neonates receiving lamivudine with or without [[zidovudine]] for the first week of life following maternal treatment starting at Week 38 or 36 of gestation. Selected adverse reactions reported in these neonates included increased [[liver function tests]], [[anemia]], [[diarrhea]], [[electrolyte disturbances]], [[hypoglycemia]], [[jaundice]] and [[hepatomegaly]], [[rash]], [[respiratory infections]], and [[sepsis]]; 3 neonates died (1 from [[gastroenteritis]] with [[acidosis]] and [[convulsions]], 1 from traumatic injury, and 1 from unknown causes). Two other nonfatal [[gastroenteritis]] or [[diarrhea]] cases were reported, including 1 with [[convulsions]]; 1 infant had transient [[renal insufficiency]] associated with [[dehydration]]. The absence of control groups limits assessments of causality, but it should be assumed that perinatally exposed infants may be at risk for adverse reactions comparable to those reported in pediatric and adult [[HIV-1]]-infected patients treated with lamivudine-containing combination regimens. Long-term effects of in [[utero]] and infant lamivudine exposure are not known. | Limited short-term safety information is available from 2 small, uncontrolled trials in South Africa in neonates receiving lamivudine with or without [[zidovudine]] for the first week of life following maternal treatment starting at Week 38 or 36 of gestation. Selected adverse reactions reported in these neonates included increased [[liver function tests]], [[anemia]], [[diarrhea]], [[electrolyte disturbances]], [[hypoglycemia]], [[jaundice]] and [[hepatomegaly]], [[rash]], [[respiratory infections]], and [[sepsis]]; 3 neonates died (1 from [[gastroenteritis]] with [[acidosis]] and [[convulsions]], 1 from traumatic injury, and 1 from unknown causes). Two other nonfatal [[gastroenteritis]] or [[diarrhea]] cases were reported, including 1 with [[convulsions]]; 1 infant had transient [[renal insufficiency]] associated with [[dehydration]]. The absence of control groups limits assessments of causality, but it should be assumed that perinatally exposed infants may be at risk for adverse reactions comparable to those reported in pediatric and adult [[HIV-1]]-infected patients treated with lamivudine-containing combination regimens. Long-term effects of in [[utero]] and infant lamivudine exposure are not known. | ||
|postmarketing=Because these reactions are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. | |postmarketing=In addition to adverse reactions reported from [[clinical trials]], the following adverse reactions have been reported during postmarketing use of EPIVIR. | ||
Because these reactions are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. | |||
'''Body as a Whole''' | '''Body as a Whole''' | ||
* | *Redistribution/accumulation of body fat. | ||
'''General''' | '''General''' | ||
| Line 140: | Line 141: | ||
'''Hepatic and Pancreatic''' | '''Hepatic and Pancreatic''' | ||
*[[Lactic acidosis]] and hepatic [[steatosis]] | *[[Lactic acidosis]] and hepatic [[steatosis]] | ||
*Posttreatment exacerbation of hepatitis B | *Posttreatment exacerbation of [[hepatitis B]] | ||
'''Hypersensitivity''' | '''Hypersensitivity''' | ||
Revision as of 15:18, 6 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: RISK OF LACTIC ACIDOSIS, EXACERBATIONS OF HEPATITIS B IN CO-INFECTED PATIENTS UPON DISCONTINUATION OF LAMIVUDINE, DIFFERENT FORMULATIONS OF LAMIVUDINE.
See full prescribing information for complete Boxed Warning.
Lactic Acidosis and Severe Hepatomegaly: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including lamivudine and other antiretrovirals. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur.
Exacerbations of Hepatitis B: Severe acute exacerbations of hepatitis B have been reported in patients who are co-infected with hepatitis B virus (HBV) and human immunodeficiency virus (HIV-1) and have discontinued lamivudine. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue lamivudine and are co-infected with HIV-1 and HBV. If appropriate, initiation of anti-hepatitis B therapy may be warranted. Important Differences Among Lamivudine-Containing Products: Lamivudine tablets (used to treat HIV-1 infection) contain a higher dose of the active ingredient (lamivudine) than EPIVIR-HBV® tablets and oral solution (used to treat chronic HBV infection). Patients with HIV-1 infection should receive only dosage forms appropriate for treatment of HIV-1. |
Overview
Lamivudine is a nucleoside analogue reverse transcriptase inhibitor that is FDA approved for the treatment of HIV-1 infection. There is a Black Box Warning for this drug as shown here. Common adverse reactions include headache, nausea, malaise and fatigue, nasal signs and symptoms, diarrhea, and cough..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Human immunodeficiency virus (HIV-1) infection
- Dosage: 300 mg daily or 150 mg q12h
- In combination with other antiretroviral agents.
Chronic Hepatitis B (HBV) infection
- Dosage: 100 mg daily
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lamivudine in adult patients.
Non–Guideline-Supported Use
Posttransplant Prophylaxis for Hepatitis B
- Dosage: 100mg daily [1]
- In combination with HBIG.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Human immunodeficiency virus (HIV-1) infection
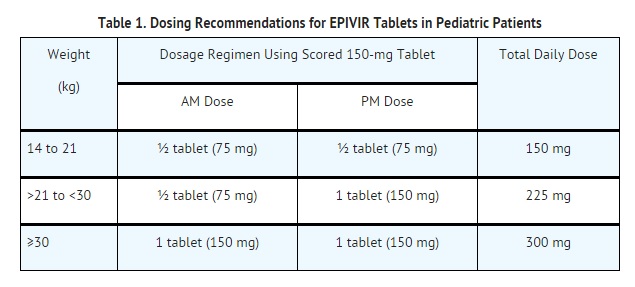
Chronic Hepatitis B (HBV) infection
- Dosage: patients aged 2 to 17 years is 3 mg per kg once daily up to a maximum daily dosage of 100 mg.
- The oral solution formulation should be prescribed for patients requiring a dosage less than 100 mg or if unable to swallow tablets.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lamivudine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Lamivudine in pediatric patients.
Contraindications
- Hypersensitivity to lamivudine or to any component of the tablets or oral solution.
Warnings
|
WARNING: RISK OF LACTIC ACIDOSIS, EXACERBATIONS OF HEPATITIS B IN CO-INFECTED PATIENTS UPON DISCONTINUATION OF LAMIVUDINE, DIFFERENT FORMULATIONS OF LAMIVUDINE.
See full prescribing information for complete Boxed Warning.
Lactic Acidosis and Severe Hepatomegaly: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including lamivudine and other antiretrovirals. Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur.
Exacerbations of Hepatitis B: Severe acute exacerbations of hepatitis B have been reported in patients who are co-infected with hepatitis B virus (HBV) and human immunodeficiency virus (HIV-1) and have discontinued lamivudine. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue lamivudine and are co-infected with HIV-1 and HBV. If appropriate, initiation of anti-hepatitis B therapy may be warranted. Important Differences Among Lamivudine-Containing Products: Lamivudine tablets (used to treat HIV-1 infection) contain a higher dose of the active ingredient (lamivudine) than EPIVIR-HBV® tablets and oral solution (used to treat chronic HBV infection). Patients with HIV-1 infection should receive only dosage forms appropriate for treatment of HIV-1. |
Lactic Acidosis and Severe Hepatomegaly With Steatosis
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including lamivudine and other antiretrovirals.
- A majority of these cases have been in women.
- Obesity and prolonged nucleoside exposure may be risk factors.
- Particular caution should be exercised when administering EPIVIR to any patient with known risk factors for liver disease; however, cases also have been reported in patients with no known risk factors. Treatment with EPIVIR should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
Patients With HIV-1 and Hepatitis B Virus Co-infection
Posttreatment Exacerbations of Hepatitis
- In clinical trials in non-HIV-1-infected patients treated with lamivudine for chronic hepatitis B, clinical and laboratory evidence of exacerbations of hepatitis have occurred after discontinuation of lamivudine.
- These exacerbations have been detected primarily by serum ALT elevations in addition to re-emergence of HBV DNA. Although most events appear to have been self-limited, fatalities have been reported in some cases.
- Similar events have been reported from postmarketing experience after changes from lamivudine-containing HIV-1 treatment regimens to non-lamivudine-containing regimens in patients infected with both HIV-1 and HBV.
- The causal relationship to discontinuation of lamivudine treatment is unknown.
- Patients should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment.
- There is insufficient evidence to determine whether re-initiation of lamivudine alters the course of posttreatment exacerbations of hepatitis.
Important Differences Among Lamivudine-Containing Products
- EPIVIR Tablets and Oral Solution contain a higher dose of the same active ingredient (lamivudine) than EPIVIR-HBV Tablets and EPIVIR-HBV Oral Solution.
- EPIVIR-HBV was developed for patients with chronic hepatitis B.
- The formulation and dosage of lamivudine in EPIVIR-HBV are not appropriate for patients co-infected with HIV-1 and HBV. Safety and efficacy of lamivudine have not been established for treatment of chronic hepatitis B in patients co-infected with HIV-1 and HBV.
- If treatment with EPIVIR-HBV is prescribed for chronic hepatitis B for a patient with unrecognized or untreated HIV-1 infection, rapid emergence of HIV-1 resistance is likely to result because of the subtherapeutic dose and the inappropriateness of monotherapy HIV-1 treatment.
- If a decision is made to administer lamivudine to patients co-infected with HIV-1 and HBV, EPIVIR Tablets, EPIVIR Oral Solution, COMBIVIR® (lamivudine/zidovudine) Tablets, EPZICOM® (abacavir sulfate and lamivudine) Tablets, or TRIZIVIR® (abacavir sulfate, lamivudine, and zidovudine) Tablets should be used as part of an appropriate combination regimen.
Emergence of Lamivudine-Resistant HBV
- In non–HIV-1-infected patients treated with lamivudine for chronic hepatitis B, emergence of lamivudine-resistant HBV has been detected and has been associated with diminished treatment response.
- Emergence of hepatitis B virus variants associated with resistance to lamivudine has also been reported in HIV-1-infected patients who have received lamivudine-containing antiretroviral regimens in the presence of concurrent infection with hepatitis B virus.
Use With Other Lamivudine- and Emtricitabine-Containing Products
- EPIVIR should not be administered concomitantly with other lamivudine-containing products including EPIVIR-HBV Tablets, EPIVIR Oral Solution, COMBIVIR (lamivudine/zidovudine) Tablets, EPZICOM (abacavir sulfate and lamivudine) Tablets, or TRIZIVIR (abacavir sulfate, lamivudine, and zidovudine) or emtricitabine-containing products, including ATRIPLA® (efavirenz, emtricitabine, and tenofovir), EMTRIVA® (emtricitabine), TRUVADA® (emtricitabine and tenofovir), or COMPLERA® (rilpivirine/emtricitabine/tenofovir).
Use With Interferon- and Ribavirin-Based Regimens
- In vitro studies have shown ribavirin can reduce the phosphorylation of pyrimidine nucleoside analogues such as lamivudine.
- Although no evidence of a pharmacokinetic or pharmacodynamic interaction (e.g., loss of HIV-1/HCV virologic suppression) was seen when ribavirin was coadministered with lamivudine in HIV-1/HCV co-infected patients, hepatic decompensation (some fatal) has occurred in HIV-1/HCV co-infected patients receiving combination antiretroviral therapy for HIV-1 and interferon alfa with or without ribavirin.
- Patients receiving interferon alfa with or without ribavirin and EPIVIR should be closely monitored for treatment-associated toxicities, especially hepatic decompensation. *Discontinuation of EPIVIR should be considered as medically appropriate.
- Dose reduction or discontinuation of interferon alfa, ribavirin, or both should also be considered if worsening clinical toxicities are observed, including hepatic decompensation (e.g., Child-Pugh >6).
Pancreatitis
- In pediatric patients with a history of prior antiretroviral nucleoside exposure, a history of pancreatitis, or other significant risk factors for the development of pancreatitis, EPIVIR should be used with caution.
- Treatment with EPIVIR should be stopped immediately if clinical signs, symptoms, or laboratory abnormalities suggestive of pancreatitis occur.
Immune Reconstitution Syndrome
- Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including EPIVIR.
- During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
- Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
Fat Redistribution
- Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy.
- The mechanism and long-term consequences of these events are currently unknown.
- A causal relationship has not been established.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults Population
Clinical Trials in HIV-1
The safety profile of EPIVIR in adults is primarily based on 3,568 HIV-1-infected subjects in 7 clinical trials.
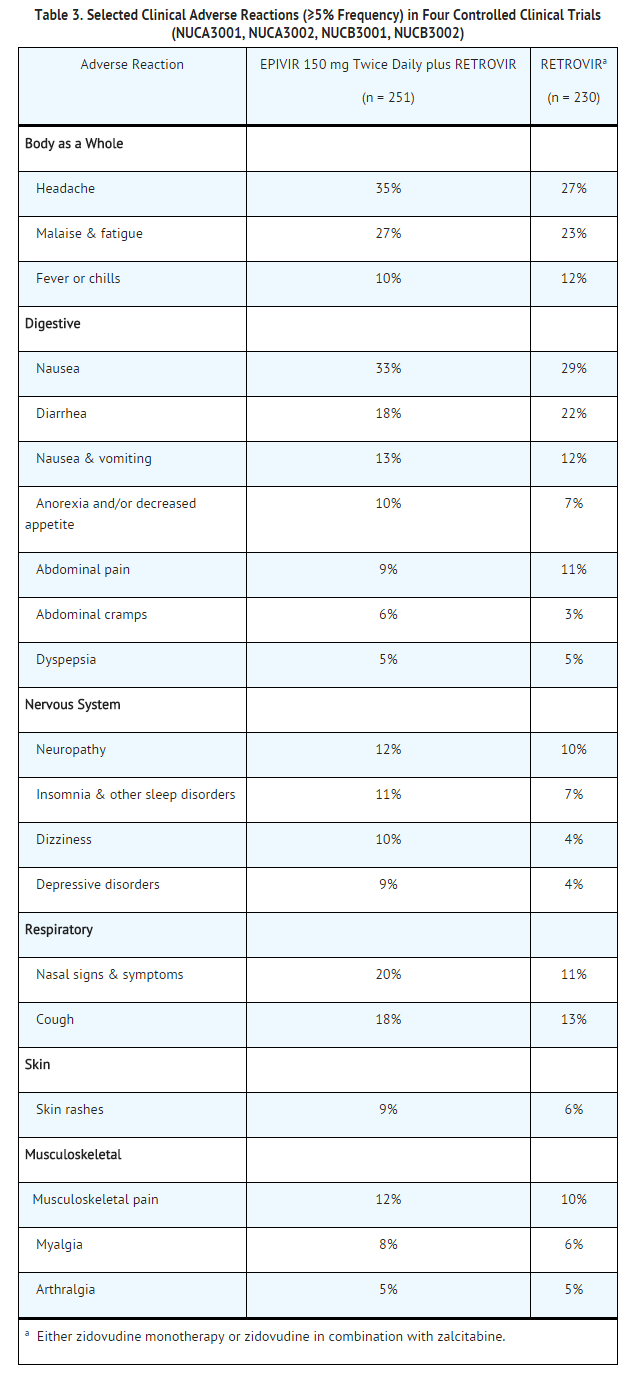
The most common adverse reactions are headache, nausea, malaise, fatigue, nasal signs and symptoms, diarrhea and cough.
Selected clinical adverse reactions in ≥5% of subjects during therapy with EPIVIR 150 mg twice daily plus RETROVIR® 200 mg 3 times daily for up to 24 weeks are listed in Table 3.
Pancreatitis
Pancreatitis was observed in 9 out of 2,613 adult subjects (0.3%) who received EPIVIR in controlled clinical trials EPV20001, NUCA3001, NUCB3001, NUCA3002, NUCB3002, and NUCB3007.
EPIVIR 300 mg Once Daily
- The types and frequencies of clinical adverse reactions reported in subjects receiving EPIVIR 300 mg once daily or EPIVIR 150 mg twice daily (in 3-drug combination regimens in EPV20001 and EPV40001) for 48 weeks were similar.
Selected laboratory abnormalities observed during therapy are summarized in Table 4.
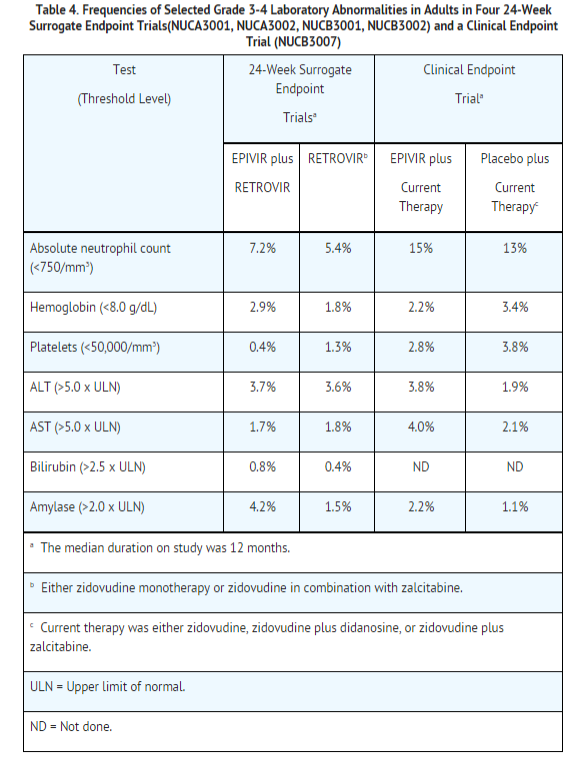
The frequencies of selected laboratory abnormalities reported subjects receiving EPIVIR 300 mg once daily or EPIVIR 150 mg twice daily (in 3-drug combination regimens in EPV20001 and EPV40001) were similar.
Pediatric Population
Clinical Trials in HIV-1
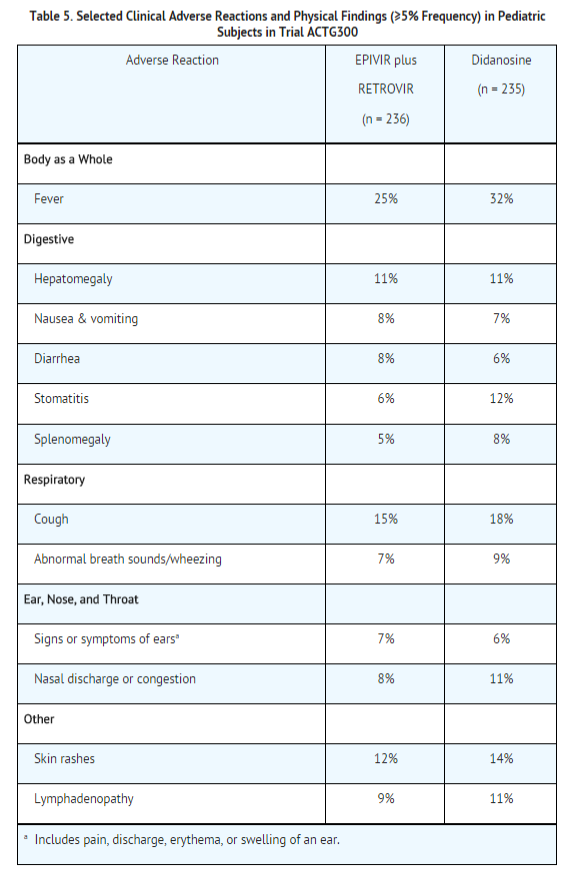
EPIVIR Oral Solution has been studied in 638 pediatric subjects aged 3 months to 18 years in 3 clinical trials. Selected clinical adverse reactions and physical findings with a ≥5% frequency during therapy with EPIVIR 4 mg/kg twice daily plus RETROVIR 160 mg/m2 3 times daily in therapy-naive (≤56 days of antiretroviral therapy) pediatric subjects are listed in Table 5.
Pancreatitis
Pancreatitis, which has been fatal in some cases, has been observed in antiretroviral nucleoside‑experienced pediatric subjects receiving EPIVIR alone or in combination with other antiretroviral agents. In an open‑label dose‑escalation trial (NUCA2002), 14 subjects (14%) developed pancreatitis while receiving monotherapy with EPIVIR. Three of these subjects died of complications of pancreatitis. In a second open‑label trial (NUCA2005), 12 subjects (18%) developed pancreatitis. In Trial ACTG300, pancreatitis was not observed in 236 subjects randomized to EPIVIR plus RETROVIR. Pancreatitis was observed in 1 subject in this trial who received open‑label EPIVIR in combination with RETROVIR and ritonavir following discontinuation of didanosine monotherapy.
Paresthesias and Peripheral Neuropathies
Paresthesias and peripheral neuropathies were reported in 15 subjects (15%) in Trial NUCA2002, 6 subjects (9%) in Trial NUCA2005, and 2 subjects (<1%) in Trial ACTG300.
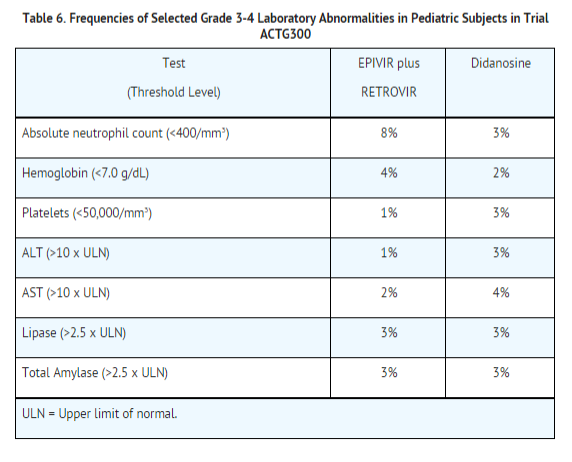
Selected laboratory abnormalities experienced by therapy‑naive (56 days of antiretroviral therapy) pediatric subjects are listed in Table 6.
Neonatal Population
Clinical Trials in HIV-1
Limited short-term safety information is available from 2 small, uncontrolled trials in South Africa in neonates receiving lamivudine with or without zidovudine for the first week of life following maternal treatment starting at Week 38 or 36 of gestation. Selected adverse reactions reported in these neonates included increased liver function tests, anemia, diarrhea, electrolyte disturbances, hypoglycemia, jaundice and hepatomegaly, rash, respiratory infections, and sepsis; 3 neonates died (1 from gastroenteritis with acidosis and convulsions, 1 from traumatic injury, and 1 from unknown causes). Two other nonfatal gastroenteritis or diarrhea cases were reported, including 1 with convulsions; 1 infant had transient renal insufficiency associated with dehydration. The absence of control groups limits assessments of causality, but it should be assumed that perinatally exposed infants may be at risk for adverse reactions comparable to those reported in pediatric and adult HIV-1-infected patients treated with lamivudine-containing combination regimens. Long-term effects of in utero and infant lamivudine exposure are not known.
Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been reported during postmarketing use of EPIVIR. Because these reactions are reported voluntarily from a population of unknown size, estimates of frequency cannot be made.
Body as a Whole
- Redistribution/accumulation of body fat.
General
Endocrine and Metabolic
Hemic and Lymphatic
- Anemia (including pure red cell aplasia and severe anemias progressing on therapy).
Hepatic and Pancreatic
- Lactic acidosis and hepatic steatosis
- Posttreatment exacerbation of hepatitis B
Hypersensitivity
Musculoskeletal
Skin
Drug Interactions
There is limited information regarding Lamivudine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Lamivudine in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lamivudine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Lamivudine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Lamivudine in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Lamivudine in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Lamivudine in geriatric settings.
Gender
There is no FDA guidance on the use of Lamivudine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lamivudine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Lamivudine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Lamivudine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Lamivudine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Lamivudine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Lamivudine Administration in the drug label.
Monitoring
There is limited information regarding Lamivudine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Lamivudine and IV administrations.
Overdosage
There is limited information regarding Lamivudine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Lamivudine Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Lamivudine Mechanism of Action in the drug label.
Structure
There is limited information regarding Lamivudine Structure in the drug label.
Pharmacodynamics
There is limited information regarding Lamivudine Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Lamivudine Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Lamivudine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Lamivudine Clinical Studies in the drug label.
How Supplied
There is limited information regarding Lamivudine How Supplied in the drug label.
Storage
There is limited information regarding Lamivudine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Lamivudine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Lamivudine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Lamivudine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Lamivudine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Lamivudine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Lamivudine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Jiang L, Yan L, Li B, Wen T, Zhao J, Jiang L; et al. (2010). "Prophylaxis against hepatitis B recurrence posttransplantation using lamivudine and individualized low-dose hepatitis B immunoglobulin". Am J Transplant. 10 (8): 1861–9. doi:10.1111/j.1600-6143.2010.03208.x. PMID 20659092.
For patient information, click here.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Lamivudine (2',3'-dideoxy-3'-thiacytidine, commonly called 3TC) is a potent nucleoside analog reverse transcriptase inhibitor (nRTI).
It is marketed by GlaxoSmithKline with the brand names Epivir and Epivir-HBV.
Lamivudine has been used for treatment of chronic hepatitis B at a lower dose than for treatment of HIV. It improves the seroconversion of e-antigen positive hepatitis B and also improves histology staging of the liver. Long term use of lamivudine unfortunately leads to emergence of a resistant hepatitis B virus (YMDD) mutant. Despite this, lamivudine is still used widely as it is well tolerated.
Category
Antiretroviral
US Brand Names
EPIVIR®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Mechanism of Action
Intracellularly, lamivudine is phosphorylated to its active 5′-triphosphate metabolite, lamivudine triphosphate (3TC-TP). The principal mode of action of 3TC-TP is the inhibition of HIV-1 reverse transcriptase (RT) via DNA chain termination after incorporation of the nucleotide analogue into viral DNA. 3TC-TP is a weak inhibitor of mammalian DNA polymerases α, β, and γ.