Iodothyronine deiodinase: Difference between revisions
(→Disease relevance: Spelling) |
Matt Pijoan (talk | contribs) m (1 revision imported) |
(No difference)
| |
Latest revision as of 06:19, 10 January 2019
| Type I thyroxine 5'-deiodinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.21.99.4 | ||||||||
| CAS number | 70712-46-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Type II thyroxine 5-deiodinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.21.99.3 | ||||||||
| CAS number | 74506-30-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Type III thyroxine 5-deiodinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| File:4TR3.png Mouse iodothyronine deiodinase 3 catalytic core rendered from PDB entry 4TR3 [1] | |||||||||
| Identifiers | |||||||||
| EC number | 1.97.1.11 | ||||||||
| CAS number | 74506-30-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Iodothyronine deiodinases (EC 1.21.99.4 and EC 1.21.99.3) are a subfamily of deiodinase enzymes important in the activation and deactivation of thyroid hormones. Thyroxine (T4), the precursor of 3,5,3'-triiodothyronine (T3) is transformed into T3 by deiodinase activity. T3, through binding a nuclear thyroid hormone receptor, influences the expression of genes in practically every vertebrate cell.[2][3] Iodothyronine deiodinases are unusual in that these enzymes contain selenium, in the form of an otherwise rare amino acid selenocysteine.[4][5][6]
These enzymes are not to be confused with the iodotyrosine deiodinases that are also deiodinases, but not members of the iodothyronine family. The iodotyrosine deiodinases (unlike the iodothyronine deiodinases) do not use selenocysteine or selenium. The iodotyrosine enzymes work on iodinated single tyrosine residue molecules to scavenge iodine, and do not use as substrates the double-tyrosine residue molecules of the various iodothyronines.
Activation and inactivation
In tissues, deiodinases can either activate or inactivate thyroid hormones:
- Activation occurs by conversion of the prohormone thyroxine (T4) to the active hormone triiodothyronine (T3) through the removal of an iodine atom on the outer ring.
- Inactivation of thyroid hormones occurs by removal of an iodine atom on the inner ring, which converts thyroxine to the inactive reverse triiodothyronine (rT3), or which converts the active triiodothyronine to diiodothyronine (T2).
The major part of thyroxine deiodination occurs within the cells.
Deionidase 2 activity can be regulated by ubiquitination:
- The covalent attachment of ubiquitin inactivates D2 by disrupting dimerization and targets it to degradation in the proteosome.[7]
- Deubiquitination removing ubiquitin from D2 restores its activity and prevents proteosomal degradation.[7]
- The Hedgehog cascade acts to increase D2 ubiquitination through WSB1 activity, decreasing D2 activity.[7][8]
D-propranolol inhibits thyroxine deiodinase, thereby blocking the conversion of T4 to T3, providing some though minimal therapeutic effect.[citation needed]
Reactions
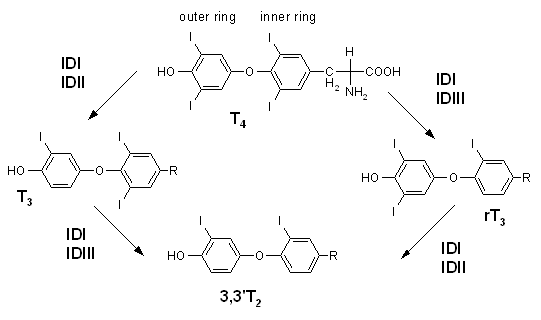 |
Structure
The three deiodinase enzymes share certain structural features in common although their sequence identity is lower than 50%. Each enzyme weighs between 29 and 33kDa.[7] Deiodinases are dimeric integral membrane proteins with single transmembrane segments and large globular heads (see below).[9] They share a TRX fold that contains the active site including the rare selenocysteine amino acid and two histidine residues.[7][10] Selenocysteine is coded by a UGA codon, which generally signifies termination of a peptide through a stop codon. In point mutation experiments with Deiodinase 1 changing UGA to the stop codon TAA resulted in a complete loss of function, while changing UGA to cysteine (TGT) caused the enzyme to operate at around 10% normal efficiency.[11] In order for UGA to be read as a selenocysteine amino acid instead of a stop codon, it is necessary that a downstream stem loop sequence, the selenocysteine insertion sequence (SECIS), be present to bind with SECIS binding protein-2 (SBP-2), which binds with elongation factor EFsec.[7] The translation of selenocysteine is not efficient,[12] even though it is important to the functioning of the enzyme. Deiodinase 2 is localized to the ER membrane while Deiodinase 1 and 3 are found in the plasma membrane.[7]
The related catalytic domains of Deiodinases 1-3 feature a thioredoxine-related peroxiredoxin fold.[13] The enzymes catalyze a reductive elimination of iodine, thereby oxidizing themselves similar to Prx, followed by a reductive recycling of the enzyme.
Types
| Type I iodothyronine deiodinase | |
|---|---|
| Identifiers | |
| Symbol | DIO1 |
| Alt. symbols | TXDI1 |
| Entrez | 1733 |
| HUGO | 2883 |
| OMIM | 147892 |
| RefSeq | NM_000792 |
| UniProt | P49895 |
| Other data | |
| EC number | 1.21.99.3 |
| Locus | Chr. 1 p32-p33 |
| Type II iodothyronine deiodinase | |
|---|---|
| Identifiers | |
| Symbol | DIO2 |
| Alt. symbols | TXDI2, SelY |
| Entrez | 1734 |
| HUGO | 2884 |
| OMIM | 601413 |
| RefSeq | NM_000793 |
| UniProt | Q92813 |
| Other data | |
| EC number | 1.21.99.4 |
| Locus | Chr. 14 q24.2-24.3 |
| Type III iodothyronine deiodinase | |
|---|---|
| Identifiers | |
| Symbol | DIO3 |
| Alt. symbols | TXDI3 |
| Entrez | 1735 |
| HUGO | 2885 |
| OMIM | 601038 |
| PDB | 4TR3 |
| RefSeq | NM_001362 |
| UniProt | P55073 |
| Other data | |
| EC number | 1.97.1.11 |
| Locus | Chr. 14 q32 |
In most vertebrates, there are three types of enzymes that can deiodinate thyroid hormones:
| Type | Location | Function |
| type I (DI) | is commonly found in the liver and kidney | DI can deiodinate both rings[14] |
| type II deiodinase (DII) | is found in the heart, skeletal muscle, CNS, fat, thyroid, and pituitary[15] | DII can only deiodinate the outer ring of the prohormone thyroxine and is the major activating enzyme (the already inactive reverse triiodothyronine is also degraded further by DII) |
| type III deiodinase (DIII) | found in the fetal tissue and the placenta; also present throughout the brain, except in the pituitary[16] | DIII can only deiodinate the inner ring of thyroxine or triiodothyronine and is the major inactivating enzyme |
Function
Deiodinase 1 both activates T4 to produce T3 and inactivates T4. Besides its increased function in producing extrathyroid T3 in patients with hyperthyroidism, its function is less well understood than D2 or D3 [2][7] Deiodinase 2, located in the ER membrane, converts T4 into T3 and is a major source of the cytoplasmic T3 pool.[2] Deiodinase 3 prevents T4 activation and inactivates T3.[9] D2 and D3 are important in homeostatic regulation in maintaining T3 levels at the plasma and cellular levels. In hyperthyroidism D2 is down regulated and D3 is upregulated to clear extra T3, while in hypothyroidism D2 is upregulated and D3 is downregulated to increase cytoplasmic T3 levels.[2][7]
Serum T3 levels remain fairly constant in healthy individuals, but D2 and D3 can regulate tissue specific intracellular levels of T3 to maintain homeostasis since T3 and T4 levels may vary by organ. Deiodinases also provide spatial and temporal developmental control of thyroid hormone levels. D3 levels are highest early in development and decrease over time, while D2 levels are high at moments of significant metamorphic change in tissues. Thus D2 enables production of sufficient T3 at necessary time points while D3 may shield tissue from overexposure to T3.[12]
Deiodinase 2 also plays a significant role in thermogenesis in brown adipose tissue (BAT). In response to sympathetic stimulation, dropping temperature, or overfeeding BAT, D2 increases oxidation of fatty acids and uncouples oxidative phosphorylation via uncoupling protein, causing mitochondrial heat production. D2 increases during cold stress in BAT and increases intracellular T3 levels. In D2 deficient models, shivering is a behavioral adaptation to the cold. However, heat production is much less efficient than uncoupling lipid oxidation.[17][18]
Disease relevance
In cardiomyopathy the heart reverts to a fetal gene programming due to the overload of the heart. Like during fetal development, thyroid hormone levels are low in the overloaded heart tissue in a local hypothyroid state, with low levels of deiodinase 1 and deiodinase 2. Although deiodinase 3 levels in a normal heart are generally low, in cardiomyopathy deiodinase 3 activity is increased to decrease energy turnover and oxygen consumption.[7]
Hypothyroidism is a disease diagnosed by decreased levels of serum thyroxine (T4). Presentation in adults leads to decreased metabolism, increased weight gain, and neuropsychiatric complications.[19] During development, hypothyroidism is considered more severe and leads to neurotoxicity as cretinism or other human cognitive disorders,[20] altered metabolism and underdeveloped organs. Medication and environmental exposures can result in hypothyroidism with changes in deiodinase enzyme activity. The drug iopanoic acid (IOP) decreased cutaneous cell proliferation by inhibition of deiodinase enzyme type 1 or 2 reducing the conversion of T4 to T3. The environmental chemical DE-71, a PBDE pentaBDE brominated flame retardant decreased hepatic deiodinase I transcription and enzyme activity in neonatal rats with hypothyroidism.[21]
Quantifying enzyme activity
In vitro, including cell culture experiments, deiodination activity is determined by incubating cells or homogenates with high amounts of labeled thyroxine (T4) and required cosubstrates. As a measure of deiodination, the production of radioactive iodine and other physiological metabolites, in particular T3 or reverse T3, are determined and expressed (e.g. as fmol/mg protein/minute).[22][23]
In vivo, deiodination activity is estimated from equilibrium levels of free T3 and free T4. A simple approximation is T3/T4 ratio,[24] a more elaborate approach is calculating sum activity of peripheral deiodinases (GD) from free T4, free T3 and parameters for protein binding, dissociation and hormone kinetics.[25][26] In atypical cases, this last approach can benefit from measurements of TBG, but usually only requires measurement of TSH, fT3 and fT4, and as such has no added laboratory requirements besides the measurement of the same.
See also
- Iodotyrosine deiodinase
- Selenium, section Evolution in biology
References
- ↑ Schweizer U, Schlicker C, Braun D, Kohrle J, Steegborn C (Jul 2014). "Crystal structure of mammalian selenocysteine-dependent iodothyronine deiodinase suggests a peroxiredoxin-like catalytic mechanism". Proc. Natl. Acad. Sci. USA. 111: 10526–31. doi:10.1073/pnas.1323873111. PMC 4115520. PMID 25002520.
- ↑ 2.0 2.1 2.2 2.3 Bianco AC, Kim BW (October 2006). "Deiodinases: implications of the local control of thyroid hormone action". J. Clin. Invest. 116 (10): 2571–9. doi:10.1172/JCI29812. PMC 1578599. PMID 17016550.
- ↑ Wu Y, Koenig RJ (August 2000). "Gene regulation by thyroid hormone". Trends Endocrinol. Metab. 11 (6): 207–11. doi:10.1016/s1043-2760(00)00263-0. PMID 10878749.
- ↑ Köhrle J (January 2000). "The selenoenzyme family of deiodinase isozymes controls local thyroid hormone availability". Rev Endocr Metab Disord. 1 (1–2): 49–58. doi:10.1023/A:1010012419869. PMID 11704992.
- ↑ Köhrle J (May 1999). "Local activation and inactivation of thyroid hormones: the deiodinase family". Mol. Cell. Endocrinol. 151 (1–2): 103–19. doi:10.1016/S0303-7207(99)00040-4. PMID 10411325.
- ↑ Köhrle J (December 2000). "The deiodinase family: selenoenzymes regulating thyroid hormone availability and action". Cell. Mol. Life Sci. 57 (13–14): 1853–63. doi:10.1007/PL00000667. PMID 11215512.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 7.9 Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC (December 2008). "Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling". Endocr. Rev. 29 (7): 898–938. doi:10.1210/er.2008-0019. PMC 2647704. PMID 18815314.
- ↑ Dentice M, Bandyopadhyay A, Gereben B, Callebaut I, Christoffolete MA, Kim BW, Nissim S, Mornon JP, Zavacki AM, Zeöld A, Capelo LP, Curcio-Morelli C, Ribeiro R, Harney JW, Tabin CJ, Bianco AC (July 2005). "The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate". Nat. Cell Biol. 7 (7): 698–705. doi:10.1038/ncb1272. PMC 1761694. PMID 15965468.
- ↑ 9.0 9.1 Bianco AC. "Thyroid hormone action starts and ends by deiodination". Bianco Lab & The University of Miami. Retrieved 2011-05-08.
- ↑ Valverde C, Croteau W, Lafleur GJ, Orozco A, Germain DL (February 1997). "Cloning and expression of a 5'-iodothyronine deiodinase from the liver of Fundulus heteroclitus". Endocrinology. 138 (2): 642–8. doi:10.1210/en.138.2.642. PMID 9002998.
- ↑ Berry MJ, Banu L, Larsen PR (January 1991). "Type I iodothyronine deiodinase is a selenocysteine-containing enzyme". Nature. 349 (6308): 438–40. doi:10.1038/349438a0. PMID 1825132.
- ↑ 12.0 12.1 St Germain DL, Galton VA (August 1997). "The deiodinase family of selenoproteins". Thyroid. 7 (4): 655–68. doi:10.1089/thy.1997.7.655. PMID 9292958.
- ↑ Schweizer U, Schlicker C, Braun D, Köhrle J, Steegborn C (Jul 2014). "Crystal structure of mammalian selenocysteine-dependent iodothyronine deiodinase suggests a peroxiredoxin-like catalytic mechanism". Proc. Natl. Acad. Sci. USA. 111: 10526–31. doi:10.1073/pnas.1323873111. PMC 4115520. PMID 25002520.
- ↑ Moreno M, Berry MJ, Horst C, Thoma R, Goglia F, Harney JW, Larsen PR, Visser TJ (May 1994). "Activation and inactivation of thyroid hormone by type I iodothyronine deiodinase". FEBS Lett. 344 (2–3): 143–6. doi:10.1016/0014-5793(94)00365-3. PMID 8187873.
- ↑ Holtorf K (2012). "Deiodinases". National Academy of Hypothyroidism.
- ↑ Kaplan MM (March 1984). "The role of thyroid hormone deiodination in the regulation of hypothalamo-pituitary function". Neuroendocrinology. 38 (3): 254–60. doi:10.1159/000123900. PMID 6371572.
- ↑ Bianco AC, Silva JE (January 1987). "Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue". J. Clin. Invest. 79 (1): 295–300. doi:10.1172/JCI112798. PMC 424048. PMID 3793928.
- ↑ de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC (November 2001). "The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue". J. Clin. Invest. 108 (9): 1379–85. doi:10.1172/JCI13803. PMC 209445. PMID 11696583.
- ↑ Kirkegaard C, Faber J (1998). "The role of thyroid hormones in depression". Eur J Endocrinol. 138 (1): 1–9. doi:10.1530/eje.0.1380001. PMID 9461307.
- ↑ Berbel P, Navarro D, Ausó E, Varea E, Rodríguez AE, Ballesta JJ, Salinas M, Flores E, Faura CC, de Escobar GM (2010). "Role of late maternal thyroid hormones in cerebral cortex development: an experimental model for human prematurity". Cereb Cortex. 20 (6): 1462–75. doi:10.1093/cercor/bhp212. PMC 2871377. PMID 19812240.
- ↑ Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PR, Birnbaum LS (January 2009). "Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups". Toxicol. Sci. 107 (1): 27–39. doi:10.1093/toxsci/kfn230. PMC 2638650. PMID 18978342.
- ↑ Steinsapir J, Harney J, Larsen PR (December 1998). "Type 2 iodothyronine deiodinase in rat pituitary tumor cells is inactivated in proteasomes". J. Clin. Invest. 102 (11): 1895–9. doi:10.1172/JCI4672. PMC 509140. PMID 9835613.
- ↑ Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FW, Crescenzi A, da-Silva WS, Harney J, Engel FB, Obregon MJ, Larsen PR, Bianco AC, Huang SA (March 2008). "Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats". J. Clin. Invest. 118 (3): 975–83. doi:10.1172/JCI32824. PMC 2230657. PMID 18259611.
- ↑ Mortoglou A, Candiloros H (2004). "The serum triiodothyronine to thyroxine (T3/T4) ratio in various thyroid disorders and after Levothyroxine replacement therapy". Hormones (Athens). 3 (2): 120–6. doi:10.14310/horm.2002.11120. PMID 16982586.
- ↑ Dietrich JW (2002). Der Hypophysen-Schilddrüsen-Regelkreis. Berlin, Germany: Logos-Verlag Berlin. ISBN 978-3-89722-850-4. OCLC 50451543. 3897228505.
- ↑ Rosolowska-Huszcz D, Kozlowska L, Rydzewski A (August 2005). "Influence of low protein diet on nonthyroidal illness syndrome in chronic renal failure". Endocrine. 27 (3): 283–8. doi:10.1385/ENDO:27:3:283. PMID 16230785.
Further reading
- Heinrich P, Löffler G, Petrides PE (2006). Biochemie und Pathobiochemie (Springer-Lehrbuch) (German Edition) (in German). Berlin: Springer. pp. 847–861. ISBN 3-540-32680-4.
External links
- Deiodinase at the US National Library of Medicine Medical Subject Headings (MeSH)
- Pages with broken file links
- All articles with unsourced statements
- Articles with unsourced statements from May 2014
- Articles with invalid date parameter in template
- Protein pages needing a picture
- Genes on human chromosome 1
- Genes on human chromosome 14
- CS1 maint: Unrecognized language
- Portal templates with all redlinked portals
- EC 1.21.99
- Selenoproteins