|
|
| (80 intermediate revisions by 17 users not shown) |
| Line 1: |
Line 1: |
| {{Infobox Disease | | | __NOTOC__ |
| Name = Hepatocellular carcinoma |
| | {{Infobox_Disease |
| Image = Hepatocellular carcinoma 001.jpg |
| | | Name = Hepatocellular carcinoma |
| Caption = Hepatocellular Carcinoma, Hepatitis C positive. Tumor growth in portal vein |
| | | Image = Hepatocellular carcinoma 1.jpg |
| ICD10 = {{ICD10|C|22|0|C|22}} |
| | | Caption = Hepatocellular carcinoma. <br> <small> "https://commons.wikimedia.org/wiki/File%3AHepatocellular_carcinoma_1.jpg" Via Wikimedia Commons </small> |
| ICD9 = {{ICD9|155}} |
| | |}} |
| ICDO = 8170/3 |
| | {{Hepatocellular carcinoma}} |
| MedlinePlus = 000280 |
| |
| eMedicineSubj = med |
| |
| eMedicineTopic = 787 |
| |
| }} | |
| {{SI}} | |
| {{CMG}}
| |
|
| |
|
| {{Editor Help}} | | '''For patient information click [[{{PAGENAME}} (patient information)|here]]''' |
|
| |
|
| ==Overview==
| | {{CMG}}; {{AE}} {{SH}}, {{MJK}} |
|
| |
|
| '''Hepatocellular carcinoma''' (HCC, also called '''hepatoma''') is a primary [[cancer|malignancy]] (cancer) of the [[liver]]. Most cases of HCC are secondary to either a viral [[hepatitis|hepatitide]] infection ([[hepatitis B]] or [[hepatitis C|C]]) or [[cirrhosis]] ([[alcoholism]] being the most common cause of hepatic cirrhosis).<ref name=Robbins_2005>{{cite book | author = Kumar V, Fausto N, Abbas A (editors) | title = Robbins & Cotran Pathologic Basis of Disease | edition = 7th | pages= pp. 914–7 | publisher = Saunders | year = 2003 | id = ISBN 978-0-721-60187-8 }}</ref> In countries where hepatitis is not [[Endemic (epidemiology)|endemic]], most [[malignant]] cancers in the liver are not primary HCC but [[metastasis]] (spread) of cancer from elsewhere in the body, e.g. [[colorectal cancer|the colon]]. Treatment options of HCC and prognosis are dependent on many factors but especially on [[tumor]] size and [[staging (pathology)|staging]].
| | {{SK}} Hepatoma; Malignant hepatoma; Liver cancer; Malignant liver tumor; Primary liver cell carcinoma; Hepatic cancer |
|
| |
|
| Outside of the West, the usual outcome is poor, because only 10 - 20% of hepatocellular carcinomas can be removed completely using surgery. If the cancer cannot be completely removed, the disease is usually deadly within 3 to 6 months [http://www.nlm.nih.gov/medlineplus/ency/article/000280.htm ]. This is partially due to late presentation with large tumours, but also the lack of medical expertise and facilities. This is a rare tumor in the United States.
| | ==[[Hepatocellular carcinoma overview|Overview]]== |
|
| |
|
| == Epidemiology == | | ==[[Hepatocellular carcinoma historical perspective|Historical perspective]]== |
|
| |
|
| HCC is the 5th most common tumor worldwide.
| | ==[[Hepatocellular carcinoma classification|Classification]]== |
| The epidemiology of HCC exhibits two main patterns, one in North America and Western Europe and another in non-Western countries, such as those in sub-Saharan Africa, Central Asia and Southeast Asia, and the Amazon basin. Males are affected more than females usually and it is more common between the 3rd and 5th decades of life<ref name=Robbins_2005 /> Hepatocellular carcinoma causes 662,000 deaths worldwide per year.<ref name="WHO">{{cite web | last = | first = | authorlink = | coauthors = | title =Cancer | work = | publisher =World Health Organization | date =February 2006 | url =http://www.who.int/mediacentre/factsheets/fs297/en/ | format = | doi = | accessdate =2007-05-24 }}</ref>
| |
|
| |
|
| === Non-Western Countries === | | ==[[Hepatocellular carcinoma pathophysiology|Pathophysiology]]== |
|
| |
|
| In some parts of the world—such as sub-Saharan Africa and Southeast Asia—HCC is the most common cancer, generally affecting men more than women, and with an age of onset between late teens and 30s. This variability is in part due to the different patterns of [[hepatitis B]] transmission in different populations - infection at or around birth predispose to earlier cancers than if people are infected later. The time between hepatitis B infection and development into HCC can be years even decades, but from diagnosis of HCC to death the average survival period is only 5.9 months, according to one Chinese study during the 1970-80s, or 3 months ([[median]] survival time) in Sub-Saharan Africa according to Manson's textbook of tropical diseases. HCC is one of the deadliest cancers in China. Food infected with ''[[Aspergillus flavus]]'' (especially [[peanut]]s and corns stored during prolonged wet seasons) which produces [[aflatoxin]], poses another risk factor for HCC.
| | ==[[Hepatocellular carcinoma causes|Causes]]== |
|
| |
|
| === North America and Western Europe === | | ==[[Hepatocellular carcinoma differential diagnosis|Differentiating Hepatocellular carcinoma from other Diseases]]== |
|
| |
|
| Most malignant tumors of the liver discovered in Western patients are [[metastasis|metastases]] (spread) from tumors elsewhere.<ref name=Robbins_2005 /> In the West, HCC is generally seen as rare cancer, normally of those with pre-existing liver disease. It is often detected by ultrasound screening, and so can be discovered by health-care facilities much earlier than in developing regions such as Sub-Saharan Africa.
| | ==[[Hepatocellular carcinoma epidemiology and demographics|Epidemiology & Demographics]]== |
|
| |
|
| Acute and chronic hepatic [[porphyrias]] (acute intermittent [[porphyria]], [[porphyria cutanea tarda]], [[hereditary coproporphyria]], [[variegate porphyria]]) and tyrosinemia type I are risk factors for hepatocellular carcinoma. The diagnosis of an acute hepatic porphyria (AIP, HCP, VP) should be sought in patients with hepatocellular carcinoma without typical risk factors of hepatitis B or C, alcoholic liver cirrhosis or hemochromatosis. Both active and latent genetic carriers of acute hepatic porphyrias
| | ==[[Hepatocellular carcinoma risk factors|Risk factors]]== |
| are at risk for this cancer, although latent genetic carriers have developed the cancer at a later age than those with classic symptoms. Patients with acute hepatic porphyrias should be monitored for hepatocellular carcinoma.
| |
|
| |
|
| ==Pathological Images== | | ==[[Hepatocellular carcinoma screening|Screening]]== |
|
| |
|
| | ==[[Hepatocellular carcinoma natural history|Natural History, Complications & Prognosis]]== |
|
| |
|
| <gallery>
| | ==Diagnosis== |
| Image:hepatoma_lo.jpg|Hepatocelluler carcinoma. The image shows a longitudinal slice taken through the full length of the liver. <br> (Courtesy of Ed Uthman, MD)
| | [[Hepatocellular carcinoma staging | Staging]] | [[Hepatocellular carcinoma Diagnostic study of choice| Diagnostic Study of Choice]] | [[Hepatocellular carcinoma history and symptoms| History and Symptoms]] | [[Hepatocellular carcinoma physical examination | Physical Examination]] | [[Hepatocellular carcinoma laboratory tests|Lab Studies]] | [[Hepatocellular carcinoma electrocardiogram|Electrocardiogram]] | [[Hepatocellular carcinoma chest x ray|Chest X Ray]] | [[Hepatocellular carcinoma MRI|MRI]] | [[Hepatocellular carcinoma CT|CT]] | [[Hepatocellular carcinoma Echocardiography or Ultrasound|Echocardiography or Ultrasound]] | [[Hepatocellular carcinoma other imaging findings|Other imaging findings]] | [[Hepatocellular carcinoma other diagnostic studies|Other diagnostic studies]] |
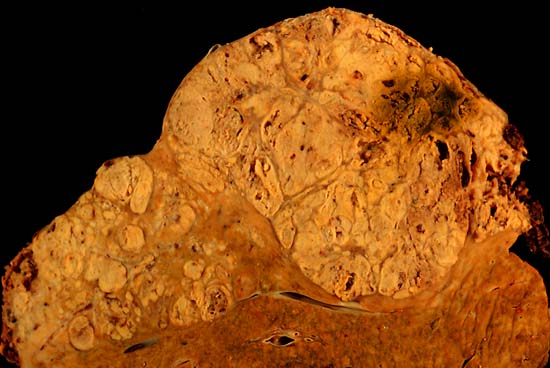
| Image:hepaca.jpg|thumb|The tumor is at the top, cirrhotic liver at the bottom, and a fibrous reaction in between. Hepatocellular carcinomas can have a variety of gross patterns, including multinodular / multifocal, such as this one. <br> (Courtesy of Ed Uthman, MD)
| |
| </gallery>
| |
|
| |
|
| == Pathogenesis == | | ==Treatment== |
| {{main|Carcinogenesis}}
| | [[Hepatocellular carcinoma medical therapy|Medical Therapy]] | [[Hepatocellular carcinoma surgery|Surgery]] | [[Hepatocellular carcinoma primary prevention|Primary prevention]] | [[Hepatocellular carcinoma secondary prevention|Secondary prevention]] | [[Hepatocellular carcinoma cost-effectiveness of therapy|Cost-Effectiveness of Therapy]] | [[Hepatocellular carcinoma future or investigational therapies|Future or Investigational Therapies]] |
| Hepatocellular carcinoma, like any other cancer, develops when there is a mutation to the cellular machinery that causes the cell to replicate at a higher rate and/or results in the cell avoiding [[apoptosis]]. In particular, chronic infections of [[Hepatitis B]] and/or [[Hepatitis C|C]] can aid the development of hepatocellular carcinoma by repeatedly causing the body's own immune system to attack the [[hepatocyte|liver cells]], some of which are infected by the virus, others merely bystanders. While this constant cycle of damage followed by repair can lead to mistakes during repair which in turn lead to carcinogenesis, this hypothesis is more applicable, at present, to Hepatitis C. In Hepatitis B, however, the integration of the viral genome into infected cells is the most consistently associated factor in malignancy. Alternatively, [[alcoholism|repeated consumption of large amounts of ethanol]] can have a similar effect. The toxin [[aflatoxin]] from certain ''[[Aspergillus]]'' species of fungus is a carcinogen and aids carcinogenesis of hepatocellular cancer by building up in the liver. The combined high prevalence of rates of aflaxtoxin and hepatitis B in countries like China and western Africa has led to relatively high rates of heptatocellular carcinoma in these regions. Other viral hepatitides such as [[hepatitis A]] have no potential to become a chronic infection and thus are not related to hepatocellular carcinoma. | | ==References== |
|
| |
|
| == Diagnosis, screening and monitoring ==
| | *[http://www.cancer.gov/cancertopics/types/liver NCI Liver Cancer Homepage] |
|
| |
|
| Hepatocellular carcinoma (HCC) most commonly appears in a patient with chronic viral hepatitis (hepatitis B or hepatitis C, 20%) or with cirrhosis (about 80%). These patients commonly undergo surveillance with [[medical ultrasonography|ultrasound]] due to the cost-effectiveness.
| |
|
| |
|
| In patients with a higher suspicion of HCC (such as rising [[alpha-fetoprotein]] and [[des-gamma carboxyprothrombin]] levels), the best method of diagnosis involves a [[computed axial tomography|CT scan]] of the abdomen using [[radiocontrast|intravenous contrast]] agent and three-phase scanning (before contrast administration, immediately after contrast administration, and again after a delay) to increase the ability of the [[radiologist]] to detect small or subtle tumors. It is important to optimize the parameters of the CT examination, because the underlying liver disease that most HCC patients have can make the findings more difficult to appreciate.
| | {{Gastroenterology}} |
| | {{Tumors}} |
| | {{Tumor morphology}} |
|
| |
|
| On CT, HCC can have three distinct patterns of growth:
| |
|
| |
|
| * A single large tumor
| |
| * Multiple tumors
| |
| * Poorly defined tumor with an infiltrative growth pattern
| |
|
| |
|
| Once imaged, diagnosis is confirmed by [[percutaneous]] [[biopsy]] and histopathologic analysis.
| | {{WikiDoc Help Menu}} |
| | | {{WikiDoc Sources}} |
| The key characteristics on CT are hypervascularity in the arterial phase scans, washout or de-enhancement in the portal and delayed phase studies, a pseudocapsule and a mosaic pattern.
| |
| Both calcifications and intralesional fat may be appreciated.
| |
| | |
| CT scans use contrast agents, which are typically iodine or barium based. Some patients are allergic to one or both of these contrast agents, most often iodine. Usually the allergic reaction is manageable and not life threating.
| |
| | |
| An alternative to a CT imaging study would be the MRI. MRI's are more expensive and not as available because fewer facilities have MRI machines. More important MRI are just beginning to be used in tumor detection and fewer radiologists are skilled at finding tumors with MRI studies when it is used as a screening device. Mostly the radiologists are using MRIs to do a secondary study to look at an area where a tumor has already been detected.
| |
| | |
| MRI's also use contrast agents. One of the best for showing details of liver tumors is very new: iron oxide nano-particles appears to give better results. The latter are absorbed by normal liver tissue, but not tumors or scar tissue. But most often MRI's use a agent based on the rare earth, gadolinium. In a very small percentage of the population, it appears that this is not a safe contrast agent. It is life threatening and several hundred people worldwide, have died out of millions who take it each year when they get MRIs. But the FDA has taken notice and has issued its highest level warning against the use of gadolinium based contrast agents in people with moderate to severe kidney disease which is what all of people who have died seem to share. In the paragraphs below are the details from the FDA website.
| |
|
| |
| ==Information for Healthcare Professionals==
| |
| | |
| Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance)
| |
| | |
| FDA ALERT [6/2006, updated 12/2006 and 5/23/2007]: This updated Alert highlights FDA’s request for addition of a boxed warning and new warnings about risk of nephrogenic systemic fibrosis (NSF) to the full prescribing information for all gadolinium-based contrast agents (GBCAs) (Magnevist, MultiHance, Omniscan, OptiMARK, ProHance). This new labeling highlights and describes the risk for NSF following exposure to a GBCA in patients with acute or chronic severe renal insufficiency (a glomerular filtration rate <30 mL/min/1.73m2) and patients with acute renal insufficiency of any severity due to the hepato-renal syndrome or in the peri-operative liver transplantation period. In these patients, avoid the use of a GBCA unless the diagnostic information is essential and not available with non-contrast enhanced magnetic resonance imaging. NSF may result in fatal or debilitating systemic fibrosis. Requested changes to GBCA product labeling are summarized below.
| |
| | |
| This information reflects FDA’s current analysis of data available to FDA concerning this drug. FDA intends to update this sheet when additional information or analyses become available.
| |
| | |
| To report any serious adverse events associated with the use of this drug, please contact the
| |
| FDA MedWatch program using the contact information at the bottom of this document.
| |
| | |
| FDA has requested manufacturers of all gadolinium-based contrast agents (GBCAs) to add a new Boxed Warning and new Warnings about Nephrogenic Systemic Fibrosis (NSF).
| |
| | |
| A new Boxed Warning and new Warnings section describe NSF, populations at risk for NSF, and advise on screening procedures, dosing and other considerations:
| |
| | |
| Boxed Warning:
| |
| | |
| * Exposure to GBCAs increases the risk for NSF in patients with:
| |
| o acute or chronic severe renal insufficiency (glomerular filtration rate <30 mL/min/1.73m2), or<br>
| |
| o acute renal insufficiency of any severity due to the hepato-renal syndrome or in the<br>perioperative liver transplantation period.
| |
| | |
| * NSF is a debilitating and sometimes fatal disease affecting the skin, muscle, and internal<br>organs.
| |
| | |
| * Avoid use of GBCAs unless the diagnostic information is essential and not available with<br>non-contrast enhanced magnetic resonance imaging (MRI).
| |
| * Screen all patients for renal dysfunction by obtaining a history and/or laboratory tests.
| |
| | |
| * When administering a GBCA, do not exceed the dose recommended in product labeling.Allow<br>sufficient time for elimination of the GBCA prior to any readministration.
| |
| | |
| ==Additional New Warnings==
| |
| | |
| * Among the factors that may increase the risk for NSF are repeated or higher than recommended<br>doses of a GBCA.
| |
| * For patients receiving hemodialysis, healthcare professionals may consider prompt<br>hemodialysis following GBCA administration in order to enhance the contrast agent's elimination. However, it is unknown if hemodialysis prevents NSF.
| |
| | |
| * Determine the renal function of patients by obtaining a medical history or conducting laboratory tests that measure renal function prior to using a GBCA.
| |
| | |
| * The risk, if any, for developing NSF among patients with mild to moderate renal insufficiency or normal renal function is unknown.
| |
| | |
| * Post-marketing reports have identified the development of NSF following single and multiple administrations of GBCAs. These reports have not always identified a specific agent. Where a specific agent was identified, the most commonly reported agent was Omniscan, followed by Magnevist and OptiMARK. NSF has also developed following the sequential administration of Omniscan and MultiHance and Omniscan and ProHance. The distribution of the number of reports for the individual GBCAs may relate to multiple factors, including more limited use of some GBCAs, under-reporting of NSF, characteristics of the agent and a lack of patients’ complete GBCA exposure history.
| |
| | |
| | |
| | |
| ==Information for the patient==
| |
| | |
| Physicians who are considering a GBCA for use in a patient who is at risk for NSF should discuss the following with the patient:
| |
| | |
| * The possibility of developing NSF, a debilitating and potentially fatal disease that involves the skin, muscle and internal organs.
| |
| | |
| * The signs and symptoms of NSF, which include:
| |
| | |
| * For the skin—burning or itching, reddened or darkened patches; and/or skin swelling, hardening and/or tightening
| |
| o For the eyes—yellow raised spots on the whites of the eyes
| |
| o For the bones, joints and muscles—joint stiffness; limited range of motion in the arms, hands, legs, or feet; pain deep in the hip bone or ribs; and/or muscle weakness
| |
| | |
| * If the patient is receiving hemodialysis: Prompt hemodialysis immediately after administering a GBCA hastens its elimination. However, whether hemodialysis prevents or reduces the risk of NSF is unknown.
| |
| | |
| * After receiving a GBCA, those patients known to be at risk for NSF require clinical follow-up and long term monitoring for the disease.
| |
| | |
| == Pathology ==
| |
| | |
| Macroscopically, liver cancer appears as a nodular or infiltrative tumor. The nodular type may be solitary (large mass) or multiple (when developed as a complication of cirrhosis). Tumor nodules are round to oval, grey or green (if the tumor produces bile), well circumscribed but not encapsulated. The diffuse type is poorly circumscribed and infiltrates the portal veins, or the hepatic veins (rarely).
| |
| | |
| Microscopically, there are four architectural and cytological types (patterns) of hepatocellular carcinoma: fibrolamellar, pseudoglandular ([[adenoid]]), [[pleomorphic]] (giant cell) and clear cell. In well differentiated forms, tumor cells resemble hepatocytes, form trabeculae, cords and nests, and may contain bile pigment in cytoplasm. In poorly differentiated forms, malignant epithelial cells are discohesive, [[pleomorphic]], [[anaplastic]], giant. The tumor has a scant stroma and central necrosis because of the poor vascularization.[http://www.pathologyatlas.ro/Hepatocellular%20Carcinoma.html 1]
| |
| | |
| == Staging and prognosis ==
| |
| | |
| Important features that guide treatment include: -
| |
| | |
| * size
| |
| * spread ([[staging (pathology)|stage]])
| |
| * involvement of liver vessels
| |
| * presence of a tumor capsule
| |
| * presence of extrahepatic metastases
| |
| * presence of daughter nodules
| |
| * vascularity of the tumor
| |
| | |
| MRI is the best imaging method to detect the presence of a tumor capsule.
| |
| | |
| == Treatment ==
| |
| | |
| * [[Liver transplantation]] to replace the liver with a cadaver liver or a live donor lobe. Historically low survival rates (20%-36%) recent improvement (61.1%; 1996-2001), likely related to adoption of Milan criteria at US transplantation centers. If the tumor disease has metastasized, the immuno-suppressant post-transplant drugs decrease the chance of survival. [http://www.nlm.nih.gov/medlineplus/livertransplantation.html NIH]
| |
| | |
| * [[Surgical resection]] to remove a tumor to treat small or slow-growing tumors if they are diagnosed early. This treatment offers the best prognosis for long-term survival but unfortunately is possible in only 10-15% of cases. Resection in cirrhotic patients carries high morbidity and mortality. [http://www.medicinenet.com/liver_resection/article.htm Medicinenet]
| |
| | |
| * Percutaneous ethanol injection (PEI) well tolerated, high RR in small (< 3 cm) solitary tumors; as of 2005, no randomized trial comparing resection to percutaneous treatments; recurrence rates similar to those for postresection.
| |
| | |
| * [[Transcatheter arterial chemoembolization]] (TACE) is usually performed in the treatment of large tumors (larger than 3 cm and less than 4 cm in diameter), most frequently by intraarterially injecting an infusion of antineoplastic agents mixed with iodized oil (such as Lipiodol). As of 2005, multiple trials show objective tumor responses and slowed tumor progression but questionable survival benefit compared to supportive care; greatest benefit seen in patients with preserved liver function, absence of vascular invasion, and smallest tumors.
| |
| | |
| *[[Sealed source radiotherapy]] can be used to destroy the tumor from within (thus minimizing exposure to healthy tissue). [[TheraSphere]] is an FDA approved treatment which has been shown in clinical trials to increase survival rate of low-risk patients. This method uses a catheter (inserted by a [[radiologist]]) to deposit radioactive particles to the area of interest.
| |
| | |
| * [[Radiofrequency ablation]] (RFA) uses high frequency radio-waves to ablate the tumour.
| |
| | |
| * Intra-arterial iodine-131–lipiodol administration Efficacy demonstrated in unresectable patients, those with portal vein [[thrombus]]. This treatment is also used as adjuvant therapy in resected patients (Lau at et, 1999). It is believed to raise the 3-year survival rate from 46 to 86%. This adjuvant therapy is in phase III clinical trials in Singapore and is available as a standard medical treatment to qualified patients in Hong Kong.
| |
| | |
| * Combined PEI and [[TACE]] can be used for tumors larger than 4 cm in diameter, although some Italian groups have had success with larger tumours using TACE alone.
| |
| | |
| * [[High intensity focused ultrasound]] (HIFU) (not to be confused with normal [[diagnostic ultrasound]]) is a new technique which uses much more powerful ultrasound to treat the tumour. Still at a very experimental stage. Most of the work has been done in China. Some early work is being done in Oxford and London in the UK.
| |
| | |
| * [[Hormonal therapy]] Antiestrogen therapy with tamoxifen studied in several trials, mixed results across studies, but generally considered ineffective Octreotide (somatostatin analogue) showed 13-month MS v 4-month MS in untreated patients in a small randomized study; results not reproduced.
| |
| | |
| * [[Chemotherapy adjuvant]]: No randomized trials showing benefit of neoadjuvant or adjuvant systemic therapy in HCC; single trial showed decrease in new tumors in patients receiving oral synthetic retinoid for 12 months after [[resection]]/[[ablation]]; results not reproduced. Clinical trials have varying results.<ref>[http://www.asco.org/ac/1,1003,_12-002626-00_18-0034-00_19-0027,00.asp] American Society of Clinical Oncology, 2005 Annual Meeting, Abstracts on Hepatobiliary Cancer</ref>
| |
| | |
| * [[Palliative]]: Regimens that included [[doxorubicin]], [[cisplatin]], [[fluorouracil]], [[interferon]], [[epirubicin]], or [[taxol]], as single agents or in combination, have not shown any survival benefit (RR, 0%-25%); a few isolated major responses allowed patients to undergo partial hepatectomy; no published results from any randomized trial of systemic chemotherapy.
| |
| | |
| * [[Cryosurgery]]: Cryosurgery is a new technique that can destroy tumors in a variety of sites (brain, breast, kidney, prostate, liver). Cryosurgery is the destruction of abnormal tissue using sub-zero temperatures. The tumor is not removed and the destroyed cancer is left to be reabsorbed by the body. Initial results in properly selected patients with unresectable liver tumors are equivalent to those of resection. Cryosurgery involves the placement of a stainless steel probe into the center of the tumor. Liquid nitrogen is circulated through the end of this device. The tumor and a half inch margin of normal liver are frozen to -190°C for 15 minutes, which is lethal to all tissues. The area is thawed for 10 minutes and then re-frozen to -190°C for another 15 minutes. After the tumor has thawed, the probe is removed, bleeding is controlled, and the procedure is complete. The patient will spend the first post-operative night in the intensive care unit and typically is discharged in 3 - 5 days. Proper selection of patients and attention to detail in performing the cryosurgical procedure are mandatory in order to achieve good results and outcomes. Frequently, cryosurgery is used in conjunction with liver resection as some of the tumors are removed while others are treated with cryosurgery. Patients may also have insertion of a hepatic intra-arterial artery catheter for post-operative chemotherapy. As with liver resection, your surgeon should have experience with cryosurgical techniques in order to provide the best treatment possible.
| |
| | |
| * [[Interventional radiology]]
| |
| | |
| ''Abbreviations: HCC, hepatocellular carcinoma; TACE, transarterial embolization/chemoembolization; PFS, progression-free survival; PS, performance status; HBV, [[hepatitis B]] virus; PEI, percutaneous ethanol injection; RR, response rate; MS, median survival.''
| |
| | |
| == Awareness ==
| |
| | |
| The Jade Ribbon Campaign is used for awareness of liver cancer in the Pacific Islands and will be introduced into America someday.
| |
| | |
| Jade is the official color of liver cancer.
| |
| | |
| == Future directions ==
| |
| | |
| Current research includes the search for the [[gene]]s that are disregulated in HCC,<ref>[http://web.archive.org/web/20060503035140/http://liver.stanford.edu/index2.asp?lang=eng&page=res_recpub Genetic research in HCC] Stanford Asian Liver Center</ref> [[protein]] markers<ref> [http://www.hmri.org/HMRI_News/Resources/newsletter_May_05.pdf Huntington Medical Research Institute News, May 2005]</ref>, and other predictive biomarkers.<ref>[http://www.jco.org/content/vol23/issue11/] ''Journal of Clinical Oncology'', Special Issue on Molecular Oncology: Receptor-Based Therapy, April 2005</ref><ref name=Lau_1999>{{cite journal |author=Lau W, Leung T, Ho S, Chan M, Machin D, Lau J, Chan A, Yeo W, Mok T, Yu S, Leung N, Johnson P |title=Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial |journal=Lancet |volume=353 |issue=9155 |pages=797-801 |year=1999 |pmid=10459961}}</ref> As similar research is yielding results in various other malignant diseases, it is hoped that identifying the aberrant genes and the resultant [[protein]]s could lead to the identification of pharmacological interventions for HCC.<ref name=Thomas_2005>{{cite journal |author=Thomas M, Zhu A |title=Hepatocellular carcinoma: the need for progress |journal=J Clin Oncol |volume=23 |issue=13 |pages=2892-9 |year=2005 |url=http://www.jco.org/cgi/content/full/23/13/2892 |pmid=15860847}}</ref>
| |
| | |
| == References ==
| |
| {{Reflist|2}}
| |
| | |
| == External links ==
| |
| | |
| * [http://www.cancer.gov/cancertopics/types/liver NCI Liver Cancer Homepage]
| |
| *[http://www.bluefaery.org Blue Faery: The Adrienne Wilson Liver Cancer Association]
| |
| | |
| {{SIB}}
| |
| {{Gastroenterology}}
| |
| {{Tumors}} | |
| {{Tumor morphology}} | |
|
| |
|
| [[Category:Gastroenterology]] | | [[Category:Gastroenterology]] |
| Line 208: |
Line 56: |
| [[Category:Hepatology]] | | [[Category:Hepatology]] |
| [[Category:Oncology]] | | [[Category:Oncology]] |
| | | [[Category:Disease]] |
| [[de:Leberzellkarzinom]]
| | [[Category:Mature chapter]] |
| [[es:Hepatocarcinoma]]
| | [[Category:Up-To-Date]] |
| [[fi:Maksasyöpä]]
| | [[Category:Oncology]] |
| [[fr:Carcinome hépatocellulaire]]
| | [[Category:Medicine]] |
| [[hr:Rak jetre]]
| | [[Category:Hepatology]] |
| [[hu:Májtumor]] | | [[Category:Gastroenterology]] |
| [[ja:肝癌]] | | [[Category:Surgery]] |
| [[la:Carcinoma hepatocellulare]] | |
| [[ko:간암]] | |
| [[nl:Leverkanker]] | |
| [[no:Leverkreft]] | |
| [[pl:Rak wątrobowokomórkowy]] | |
| [[pt:Hepatocarcinoma]] | |
| [[sv:Levercancer]]
| |
| [[zh:肝癌]]
| |
| | |
| {{WikiDoc Help Menu}}
| |
| {{WikiDoc Sources}}
| |