Glucose-6-phosphate dehydrogenase deficiency pathophysiology
|
Xyz Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Glucose-6-phosphate dehydrogenase deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
FDA on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
CDC on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
Glucose-6-phosphate dehydrogenase deficiency pathophysiology in the news |
|
Blogs on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
Risk calculators and risk factors for Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mahda Alihashemi M.D. [2]
Overview
The exact pathogenesis of [disease name] is not fully understood.
OR
It is thought that [disease name] is the result of / is mediated by / is produced by / is caused by either [hypothesis 1], [hypothesis 2], or [hypothesis 3].
OR
[Pathogen name] is usually transmitted via the [transmission route] route to the human host.
OR
Following transmission/ingestion, the [pathogen] uses the [entry site] to invade the [cell name] cell.
OR
[Disease or malignancy name] arises from [cell name]s, which are [cell type] cells that are normally involved in [function of cells].
OR
The progression to [disease name] usually involves the [molecular pathway].
OR
The pathophysiology of [disease/malignancy] depends on the histological subtype.
Pathophysiology
Physiology
The normal physiology of [name of process] can be understood as follows:
Pathogenesis
Glucose-6-phosphate dehydrogenase (G6PD) deficiency, an X-linked disorder, is the most common enzymatic disorder of red blood cells in humans, affecting more than 400 million people worldwide [1-4]. The clinical expression of G6PD variants encompasses a spectrum of hemolytic syndromes. Affected patients are most often asymptomatic, but many patients have episodic anemia, while a few have chronic hemolysis.
With most G6PD variants, hemolysis is induced in children and adults by the sudden destruction of older, more deficient erythrocytes after exposure to drugs having a high redox potential (including the antimalarial drug primaquine and certain sulfa drugs) or to fava beans, selected infections, or metabolic abnormalities (table 1). However, in the neonate with G6PD deficiency, decreased bilirubin elimination may play an important role in the development of jaundice (see 'Jaundice in neonates' below) [5,6].
Normal enzyme function and the genetics and pathophysiology of G6PD deficiency, including its possible role in protecting against severe malaria, will be reviewed here. The clinical manifestations, diagnosis, and treatment of this disorder are discussed separately. (See "Diagnosis and management of glucose-6-phosphate dehydrogenase (G6PD) deficiency".)
A historical review of the discovery of this defect, its clinical manifestations, detection, population genetics, and molecular biology, written by Dr. Ernest Beutler, a pioneer in the understanding of this disorder, is available [7].
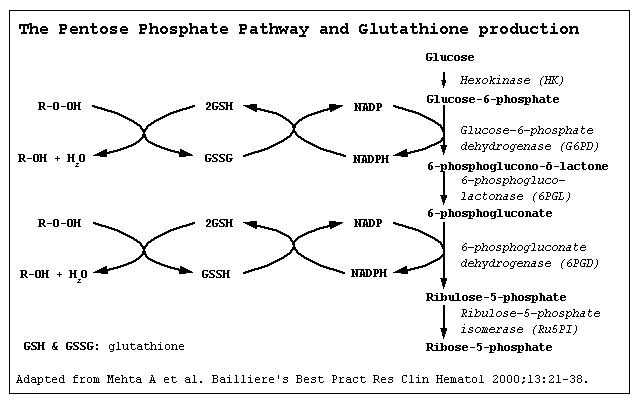
FUNCTION OF G6PD — Glucose-6-phosphate dehydrogenase catalyzes the initial step in the hexose monophosphate (HMP or pentose phosphate) shunt, oxidizing glucose-6-phosphate to 6-phosphogluconolactone and reducing nicotinamide adenine dinucleotide phosphate (NADP) to NADPH (figure 1). The HMP shunt is the only red cell source of NADPH, a cofactor important in glutathione metabolism.
The main function of the HMP shunt is to protect red blood cells against oxidative injury via the production of NADPH. Red blood cells contain relatively high concentrations of reduced glutathione (GSH), a sulfhydryl-containing tripeptide that functions as an intracellular reducing agent, thereby protecting against oxidant injury. Oxidants, such as superoxide anion (O2-) and hydrogen peroxide, are formed within red cells via reactions of hemoglobin with oxygen and can also be produced by exogenous factors such as drugs and infection. If these oxidants accumulate within red cells, hemoglobin and other proteins are oxidized (see below), leading to loss of function and cell death.
Under normal circumstances, oxidant accumulation does not occur, since these compounds are rapidly inactivated by GSH in conjunction with glutathione peroxidase. These reactions result in the conversion of GSH to oxidized glutathione (GSSG). GSH levels are restored by glutathione reductase which catalyzes the reduction of GSSG to GSH. This reaction requires the NADPH generated by G6PD.
Thus, tight coupling of the HMP shunt to glutathione metabolism is responsible for protecting intracellular proteins from oxidative injury. Almost all hemolytic episodes related to altered HMP shunt and glutathione metabolism are due to G6PD deficiency. Rarely, hemolysis results from deficiencies in GSH synthetic enzymes. (See "Disorders of the hexose monophosphate shunt and glutathione metabolism other than glucose-6-phosphate dehydrogenase deficiency".)
- The exact pathogenesis of [disease name] is not completely understood.
OR
- It is understood that G6PD deficiency is the result of reduced Glucose-6-phosphate dehydrogenase enzyme levels. G6PD deficiency is an X-linked disorder. It is the most common enzymatic disorder of red blood cells. Glucose-6-phosphate dehydrogenase enzyme oxidize glucose-6-phosphate to 6-phosphogluconolactone in pentose phosphate pathway ( HMP shunt). Glucose-6-phosphate dehydrogenase enzyme also reduces nicotinamide adenine dinucleotide phosphate (NADP) to NADPH.
- [Pathogen name] is usually transmitted via the [transmission route] route to the human host.
- Following transmission/ingestion, the [pathogen] uses the [entry site] to invade the [cell name] cell.
- [Disease or malignancy name] arises from [cell name]s, which are [cell type] cells that are normally involved in [function of cells].
- The progression to [disease name] usually involves the [molecular pathway].
- The pathophysiology of [disease/malignancy] depends on the histological subtype.
Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme in the pentose phosphate pathway (see image, also known as the HMP shunt pathway). G6PD converts glucose-6-phosphate into 6-phosphoglucono-δ-lactone and is the rate-limiting enzyme of this metabolic pathway that supplies reducing energy to cells by maintaining the level of the reduced form of the co-enzyme nicotinamide adenine dinucleotide phosphate (NADPH). The NADPH in turn maintains the supply of reduced glutathione in the cells that is used to mop up free radicals that cause oxidative damage.
The G6PD / NADPH pathway is the only source of reduced glutathione in red blood cells (erythrocytes). The role of red cells as oxygen carriers puts them at substantial risk of damage from oxidizing free radicals except for the protective effect of G6PD/NADPH/glutathione.
People with G6PD deficiency are therefore at risk of hemolytic anemia in states of oxidative stress. Oxidative stress can result from infection and from chemical exposure to medication and certain foods. Broad beans, e.g., fava beans, contain high levels of vicine, divicine, convicine and isouramil, all of which create oxidants.
When all remaining reduced glutathione is consumed, enzymes and other proteins (including hemoglobin) are subsequently damaged by the oxidants, leading to cross-bonding and protein deposition in the red cell membranes. Damaged red cells are phagocytosed and sequestered (taken out of circulation) in the spleen. The hemoglobin is metabolized to bilirubin (causing jaundice at high concentrations). The red cells rarely disintegrate in the circulation, so hemoglobin is rarely excreted directly by the kidney, but this can occur in severe cases, causing acute renal failure.
Deficiency of G6PD in the alternative pathway causes the buildup of glucose and thus there is an increase of advanced glycation endproducts (AGE). The deficiency also reduces the amount of NADPH, which is required for the formation of nitric oxide (NO). The high prevalence of diabetes mellitus type 2 and hypertension in Afro-Caribbeans in the West could be directly related to the incidence of G6PD deficiency in those populations.
Although female carriers can have a mild form of G6PD deficiency (dependent on the degree of inactivation of the unaffected X chromosome – see lyonization), homozygous females have been described; in these females there is co-incidence of a rare immune disorder termed chronic granulomatous disease (CGD).
Genetics
[Disease name] is transmitted in [mode of genetic transmission] pattern.
OR
Genes involved in the pathogenesis of [disease name] include:
- [Gene1]
- [Gene2]
- [Gene3]
OR
The development of [disease name] is the result of multiple genetic mutations such as:
- [Mutation 1]
- [Mutation 2]
- [Mutation 3]
Associated Conditions
Gross Pathology
On gross pathology, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
Microscopic Pathology
On microscopic histopathological analysis, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
References
|
Glucose-6-phosphate dehydrogenase deficiency Microchapters |
|
Differentiating Glucose-6-phosphate dehydrogenase deficiency from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Glucose-6-phosphate dehydrogenase deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
FDA on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
CDC on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
Glucose-6-phosphate dehydrogenase deficiency pathophysiology in the news |
|
Blogs on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
Directions to Hospitals Treating Glucose-6-phosphate dehydrogenase deficiency |
|
Risk calculators and risk factors for Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]; Associate Editor(s)-In-Chief: Priyamvada Singh, M.D. [4]
Please help WikiDoc by adding content here. It's easy! Click here to learn about editing.
Overview
Pathophysiology