Flecainide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 62: | Line 62: | ||
[[Category:Sodium channel blockers]] | [[Category:Sodium channel blockers]] | ||
[[Category:Cardiovascular Drugs]] | [[Category:Cardiovascular Drugs]] | ||
[[Category:Drugs]] | |||
==Dosing and Administration== | |||
<br> | |||
---- | |||
<br> | |||
<font size="4"> | |||
[[{{PAGENAME}}#FDA Package Insert Resources|FDA Package Insert Resources]] | |||
<br></font size><small>Indications, Contraindications, Side Effects, Drug Interactions, etc.</small><font size="4"><br> | |||
<br> | |||
[http://www.pace-med-apps.com/gfrcalc.htm Calculate Creatine Clearance] | |||
<br></font size><small>On line calculator of your patients Cr Cl by a variety of formulas.</small><font size="4"><br> | |||
<br> | |||
[http://home.earthlink.net/~sensei11/convert.htm Convert pounds to Kilograms] | |||
<br></font size><small>On line calculator of your patients weight in pounds to Kg for dosing estimates.</small><font size="4"><br> | |||
<br> [[{{PAGENAME}}#Publication Resources|Publication Resources]] | |||
<br></font size><small>Recent articles, WikiDoc State of the Art Review, Textbook Information</small><font size="4"><br> | |||
<br> | |||
[[{{PAGENAME}}#Trial Resources|Trial Resources]] | |||
<br></font size><small>Ongoing Trials, Trial Results</small><font size="4"><br> | |||
<br> | |||
[[{{PAGENAME}}#Guidelines & Evidence Based Medicine Resources|Guidelines & Evidence Based Medicine Resources]] | |||
<br></font size><small>US National Guidelines, Cochrane Collaboration, etc.</small><font size="4"><br> | |||
<br> | |||
[[{{PAGENAME}}#Media Resources|Media Resources]] | |||
<br></font size><small>Slides, Video, Images, MP3, Podcasts, etc.</small><font size="4"><br><br> | |||
[[{{PAGENAME}}#Patient Resources|Patient Resources]] | |||
<br></font size><small>Discussion Groups, Handouts, Blogs, News, etc.</small><font size="4"><br> | |||
<br> | |||
[[{{PAGENAME}}#International Resources|International Resources]] | |||
<br></font size><small>en Español</small><font size="4"><br> | |||
<br> | |||
---- | |||
<br> | |||
<br> | |||
<br> | |||
<br> | |||
==FDA Package Insert Resources== | |||
[[{{PAGENAME}} indications|Indications]] | |||
<br> | |||
<br> | |||
[[{{PAGENAME}} contraindications|Contraindications]] | |||
<br> | |||
<br> | |||
[[{{PAGENAME}} side effects|Side Effects]] | |||
<br> | |||
<br> | |||
[[{{PAGENAME}} drug interactions|Drug Interactions]] | |||
<br> | |||
<br> | |||
[[{{PAGENAME}} precautions|Precautions]] | |||
<br> | |||
<br> | |||
[[{{PAGENAME}} overdose|Overdose]] | |||
<br> | |||
<br> | |||
[[{{PAGENAME}} instructions for administration|Instructions for Administration]] | |||
<br> | |||
<br> | |||
[[{{PAGENAME}} how supplied|How Supplied]] | |||
<br> | |||
<br> | |||
[[{{PAGENAME}} pharmacokinetics and molecular data|Pharmacokinetics and Molecular Data]] | |||
<br> | |||
<br> | |||
[[FDA label]] | |||
<br> | |||
<br> | |||
[http://google2.fda.gov/search?q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}&x=0&y=0&client=FDA&site=FDA&lr=&proxystylesheet=FDA&output=xml_no_dtd&getfields=* FDA on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
</font size><small>[[{{PAGENAME}}#Dosing and Administration|Return to top]]</small><font size="4"> | |||
<br> | |||
<br> | |||
==Publication Resources== | |||
[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&db=pubmed&term={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}} Most Recent Articles on {{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}] | |||
<br> | |||
<br> | |||
[http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=search&db=pubmed&term={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}%20AND%20systematic%5Bsb%5D Review Articles on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=search&db=pubmed&term={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}+AND+%28%28N+Engl+J+Med%5Bta%5D%29+OR+%28Lancet%5Bta%5D%29+OR+%28BMJ%5Bta%5D%29%29 Articles on {{PAGENAME}} in N Eng J Med, Lancet, BMJ] | |||
<br> | |||
<br> | |||
[[WikiDoc state of the art review on {{PAGENAME}}|WikiDoc State of the Art Review]] | |||
<br> | |||
<br> | |||
[http://books.google.com/books?ie=UTF-8&oe=utf-8&q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}&qt_s=Search&sa=N&tab=gp Textbook Information on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
</font size><small>[[{{PAGENAME}}#Dosing and Administration|Return to top]]</small><font size="4"> | |||
<br> | |||
<br> | |||
==Trial Resources== | |||
[http://clinicaltrials.gov/search/open/condition={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}} Ongoing Trials with {{PAGENAME}} at Clinical Trials.gov] | |||
<br> | |||
<br> | |||
[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=search&db=pubmed&term={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}+AND+%28randomized+controlled+trial%5BPublication+Type%5D+OR+%28randomized%5BTitle%2FAbstract%5D+AND+controlled%5BTitle%2FAbstract%5D+AND+trial%5BTitle%2FAbstract%5D%29%29 Trial Results with {{PAGENAME}}] | |||
<br> | |||
<br> | |||
</font size><small>[[{{PAGENAME}}#Dosing and Administration|Return to top]]</small><font size="4"> | |||
<br> | |||
<br> | |||
==Guidelines & Evidence Based Medicine Resources== | |||
[http://www.guideline.gov/search/searchresults.aspx?Type=3&txtSearch={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}&num=20 US National Guidelines Clearinghouse on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=search&db=pubmed&term={{urlencode:({{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}) AND (Cochrane Database Syst Rev[ta])}} Cochrane Collaboration on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=search&db=pubmed&term={{urlencode:({{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}) AND (Cost effectiveness)}} Cost Effectiveness of {{PAGENAME}}] | |||
<br> | |||
<br> | |||
</font size><small>[[{{PAGENAME}}#Dosing and Administration|Return to top]]</small><font size="4"> | |||
<br> | |||
<br> | |||
==Media Resources== | |||
[http://www.google.com/search?q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}+ppt&ie=utf-8&oe=utf-8&aq=t&rls=org.mozilla:en-US:official&client=firefox-a Powerpoint Slides on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
[http://images.google.com/images?q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}&ie=UTF-8&oe=utf-8&rls=org.mozilla:en-US:official&client=firefox-a&um=1&sa=N&tab=wi Images of {{PAGENAME}}] | |||
<br> | |||
<br> | |||
[http://www.google.com/search?hl=en&client=firefox-a&rls=org.mozilla%3Aen-US%3Aofficial&hs=hPo&q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}+podcasts+OR+MP3&btnG=Search Podcasts & MP3s on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
[http://video.google.com/videosearch?q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}&ie=UTF-8&oe=utf-8&rls=org.mozilla:en-US:official&um=1&sa=N&tab=fv# Videos on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
</font size><small>[[{{PAGENAME}}#Dosing and Administration|Return to top]]</small><font size="4"> | |||
<br> | |||
<br> | |||
==Patient Resources== | |||
[[{{PAGENAME}} (patient information)|Patient Information from National Library of Medicine]] | |||
<br> | |||
<br> | |||
[http://www.google.com/search?hl=en&q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}+more:for_patients&cx=disease_for_patients&sa=N&oi=cooptsr&resnum=0&ct=col3&cd=1 Patient Resources on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
[http://groups.google.com/groups/search?ie=UTF-8&oe=utf-8&q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}&qt_s=Search Discussion Groups on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
[http://www.google.com/search?hl=en&client=firefox-a&rls=org.mozilla:en-US:official&q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}+more:patient_handouts&cx=disease_for_health_professionals&sa=N&oi=coopctx&resnum=0&ct=col1&cd=3 Patient Handouts on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
[http://blogsearch.google.com/blogsearch?q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}&ie=UTF-8&oe=utf-8&rls=org.mozilla:en-US:official&client=firefox-a&um=1&sa=N&tab=wb Blogs on {{PAGENAME}}] | |||
<br> | |||
<br> | |||
[http://news.google.com/news?q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}&ie=UTF-8&oe=utf-8&rls=org.mozilla:en-US:official&client=firefox-a&um=1&sa=N&tab=wn {{PAGENAME}} in the News] | |||
<br> | |||
<br> | |||
[http://finance.google.com/finance?ie=UTF-8&oe=utf-8&q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}&qt_s=Search&sa=N&tab=te {{PAGENAME}} in the Marketplace] | |||
<br> | |||
<br> | |||
</font size><small>[[{{PAGENAME}}#Dosing and Administration|Return to top]]</small><font size="4"> | |||
<br> | |||
<br> | |||
==International Resources== | |||
[http://www.google.com/search?q={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}+en+espanol&ie=utf-8&oe=utf-8&aq=t&rls=org.mozilla:en-US:official&client=firefox-a {{PAGENAME}} en Español] | |||
<br> | |||
<br> | |||
</font size><small>[[{{PAGENAME}}#Dosing and Administration|Return to top]]</small><font size="4"> | |||
<br> | |||
<br> | |||
</font size> | |||
---- | |||
{{FDA}} | |||
[[Category:Drugs]] | [[Category:Drugs]] | ||
Revision as of 12:25, 12 March 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
For patient information about Flecainide, click here.
Synonyms / Brand Names: FLECAINIDE ACETATE®
 |
Overview
Flecainide acetate (/flɛˈkeɪnaɪd/ Template:USdict) is a class Ic antiarrhythmic agent used to prevent and treat tachyarrhythmias (abnormal fast rhythms of the heart). It is used to treat a variety of cardiac arrhythmias including paroxysmal atrial fibrillation (episodic irregular heartbeat originating in the upper chamber of the heart), paroxysmal supraventricular tachycardia (episodic rapid but regular heartbeat originating in the atrium), and ventricular tachycardia (rapid rhythms of the lower chambers of the heart). Flecainide works by regulating the flow of sodium in the heart, causing prolongation of the cardiac action potential.
Flecainide is sold under the trade name Tambocor (manufactured by 3M pharmaceuticals). Flecainide went off-patent on February 10, 2004. In addition to being marketed as Tambocor, it is also available in generic version and under the trade names Almarytm, Apocard, Ecrinal, and Flécaine.
Category
Antiarrhythmic agents;Piperidines;Benzamides;Phenol ethers;Organofluorides;Sodium channel blockers;Cardiovascular Drugs
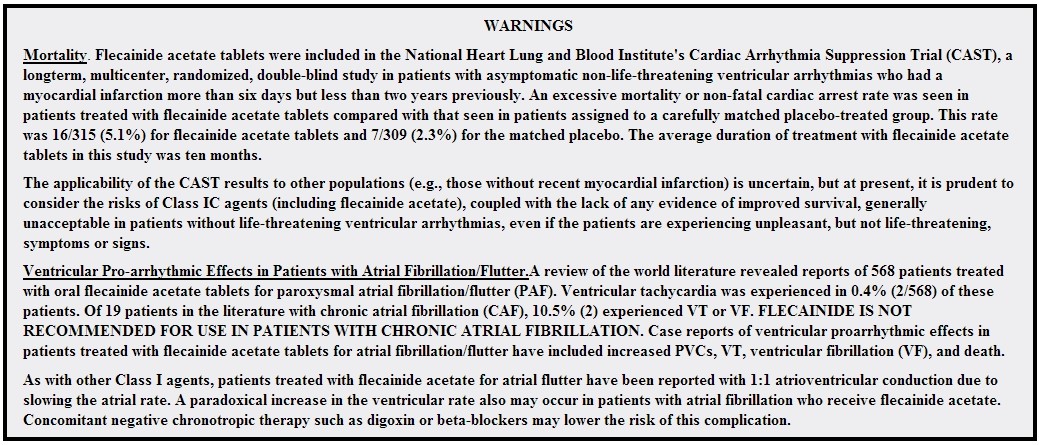
FDA Package Insert
FLECAINIDE ACETATE tablet
Indications and Usage | Dosage and Administration | Dosage Forms and Strengths | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Use in Specific Populations | Overdosage | Description | Clinical Pharmacology | Nonclinical Toxicology | Clinical Studies | How Supplied/Storage and Handling | Labels and Packages
Mechanism of Action
Flecainide works by blocking the Nav1.5 sodium channel in the heart, causing prolongation of the cardiac action potential.[1] This thereby slows conduction of the electrical impulse within the heart. The greatest effect is on the His-Purkinje system and ventricular myocardium. The effect of flecainide on the ventricular myocardium causes decreased contractility of the muscle, which leads to a decrease in the ejection fraction.
The effect of flecainide on the sodium channels of the heart increases as the heart rate increases.[2] This is known as use-dependence. This means that flecainide is potentially more useful to break a tachyarrhythmia (because it has increased effect during the fast heart rate) than to prevent a bradyarrhythmia from occurring (because of its lowered effectiveness during slower heart rates).
Interaction with Alcohl
Concomitant overdose of other drugs and/or alcohol in many instances undoubtedly contributed to the fatal outcome.
References
- ↑ Ramos E, O'leary M (2004). "State-dependent trapping of flecainide in the cardiac sodium channel". J Physiol. 560 (Pt 1): 37–49. doi:10.1113/jphysiol.2004.065003. PMC 1665201. PMID 15272045.
- ↑ Wang Z, Fermini B, Nattel S (1993). "Mechanism of flecainide's rate-dependent actions on action potential duration in canine atrial tissue". J Pharmacol Exp Ther. 267 (2): 575–81. PMID 8246130.
Template:Antiarrhythmic agents
Dosing and Administration
FDA Package Insert Resources
Indications, Contraindications, Side Effects, Drug Interactions, etc.
Calculate Creatine Clearance
On line calculator of your patients Cr Cl by a variety of formulas.
Convert pounds to Kilograms
On line calculator of your patients weight in pounds to Kg for dosing estimates.
Publication Resources
Recent articles, WikiDoc State of the Art Review, Textbook Information
Trial Resources
Ongoing Trials, Trial Results
Guidelines & Evidence Based Medicine Resources
US National Guidelines, Cochrane Collaboration, etc.
Media Resources
Slides, Video, Images, MP3, Podcasts, etc.
Patient Resources
Discussion Groups, Handouts, Blogs, News, etc.
International Resources
en Español
FDA Package Insert Resources
Indications
Contraindications
Side Effects
Drug Interactions
Precautions
Overdose
Instructions for Administration
How Supplied
Pharmacokinetics and Molecular Data
FDA label
FDA on Flecainide
Return to top
Publication Resources
Most Recent Articles on Flecainide
Review Articles on Flecainide
Articles on Flecainide in N Eng J Med, Lancet, BMJ
WikiDoc State of the Art Review
Textbook Information on Flecainide
Return to top
Trial Resources
Ongoing Trials with Flecainide at Clinical Trials.gov
Trial Results with Flecainide
Return to top
Guidelines & Evidence Based Medicine Resources
US National Guidelines Clearinghouse on Flecainide
Cochrane Collaboration on Flecainide
Cost Effectiveness of Flecainide
Return to top
Media Resources
Powerpoint Slides on Flecainide
Images of Flecainide
Podcasts & MP3s on Flecainide
Videos on Flecainide
Return to top
Patient Resources
Patient Information from National Library of Medicine
Patient Resources on Flecainide
Discussion Groups on Flecainide
Patient Handouts on Flecainide
Blogs on Flecainide
Flecainide in the News
Flecainide in the Marketplace
Return to top
International Resources
Flecainide en Español
Return to top
Adapted from the FDA Package Insert.