Emedastine
{{DrugProjectFormSinglePage |authorTag=Ammu Susheela, M.D. [1] |genericName=emedastine difumarate |aOrAn=a |drugClass=antihistamine |indicationType=treatment |indication=signs and symptoms of allergic conjunctivitis |adverseReactions=headache |blackBoxWarningTitle=ConditionName: |blackBoxWarningBody=ConditionName:
- Content
|fdaLIADAdult=* EMADINE® (emedastine difumarate ophthalmic solution) 0.05% is indicated for the temporary relief of the signs and symptoms of allergic conjunctivitis.
- EMADINE® (emedastine difumarate ophthalmic solution) 0.05% is indicated for the temporary relief of the signs and symptoms of allergic conjunctivitis.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Emedastine in adult patients.
|offLabelAdultNoGuideSupport=* Asthma; Adjunct |fdaLIADPed=There is limited information regarding FDA-Labeled Use of Emedastine in pediatric patients.
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Emedastine in pediatric patients.
|offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Emedastine in pediatric patients.
|contraindications=* EMADINE® (emedastine difumarate ophthalmic solution) is contraindicated in persons with a known hypersensitivity to emedastine difumarate or any of its components. |warnings=* FOR TOPICAL OPHTHALMIC USE ONLY - NOT FOR INJECTION OR ORAL USE. |postmarketing=* In controlled clinical studies of EMADINE® (emedastine difumarate ophthalmic solution) 0.05% lasting for 42 days, the most frequent adverse reaction was headache 11%. The following adverse experiences were reported in less than 5% of patients: Abnormal dreams, asthenia, bad taste, blurred vision, burning or stinging, corneal infiltrates, corneal staining, dermatitis, discomfort, dry eye, foreign body sensation, hyperemia, keratitis, pruritus, rhinitis, sinusitis and tearing. Some of these events were similar to the underlying disease being studied. |drugInteractions= |FDAPregCat=B |useInPregnancyFDA=* Teratology and peri- and post-natal studies have been conducted with emedastine difumarate in rats and rabbits. At 15,000 times the maximum recommended ocular human use level, emedastine difumarate was shown not to be teratogenic in rats and rabbits and no effects on peri/post-natal development were observed in rats. However, at 70,000 times the maximum recommended ocular human use level, emedastine difumarate was shown to increase the incidence of external, visceral and skeletal anomalies in rats. There are, however, no adequate and well controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. |useInPregnancyAUS=* Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Emedastine in women who are pregnant. |useInLaborDelivery=There is no FDA guidance on use of Emedastine during labor and delivery. |useInNursing=* Emedastine has been identified in breast milk in rats following oral administration. It is not known whether topical ocular administration could result in sufficient systemic absorption to produce detectable quantities in breast milk. Nevertheless, caution should be exercised when EMADINE® (emedastine difumarate ophthalmic solution) 0.05% is administered to a nursing mother. |useInPed=Safety and effectiveness in pediatric patients below the age of 3 years have not been established. |useInGeri=No overall differences in safety or effectiveness have been observed between elderly and younger patients. |useInGender=There is no FDA guidance on the use of Emedastine with respect to specific gender populations. |useInRace=There is no FDA guidance on the use of Emedastine with respect to specific racial populations. |useInRenalImpair=There is no FDA guidance on the use of Emedastine in patients with renal impairment. |useInHepaticImpair=There is no FDA guidance on the use of Emedastine in patients with hepatic impairment. |useInReproPotential=There is no FDA guidance on the use of Emedastine in women of reproductive potentials and males. |useInImmunocomp=There is no FDA guidance one the use of Emedastine in patients who are immunocompromised.
|administration=* [[Topical] |monitoring=There is limited information regarding Monitoring of Emedastine in the drug label.
|IVCompat=There is limited information regarding IV Compatibility of Emedastine in the drug label.
|overdose=* Somnolence and malaise have been reported following daily oral administration. Oral ingestion of the contents of a 15 mL DROP-TAINER® dispenser would be equivalent to 7.5 mg. In case of overdosage, treatment is symptomatic and supportive. |drugBox=
| |
Emedastine
| |
| Systematic (IUPAC) name | |
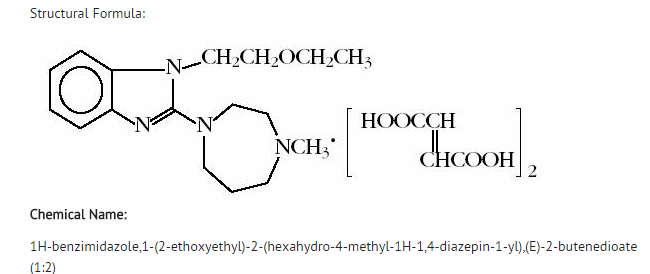
| 1-(2-ethoxyethyl)-2- (4-methyl-1,4-diazepan-1-yl)- benzoimidazole | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 302.415 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
|mechAction=* Emedastine is a relatively selective, histamine H1 antagonist. In vitro examinations of emedastine's affinity for histamine receptors (H1: Ki=1.3 nM, H2: Ki=49,067 nM, and H3: Ki=12,430 nM) demonstrate relative selectivity for the H1 histamine receptor.
- In vivo studies have shown concentration-dependent inhibition of histamine-stimulated vascular permeability in the conjunctiva following topical ocular administration. Emedastine appears to be devoid of effects on adrenergic, dopaminergic and serotonin receptors.
|structure=
|PD=There is limited information regarding Pharmacodynamics of Emedastine in the drug label.
|PK=* Following topical administration in man, emedastine was shown to have low systemic exposure. In a study involving 10 normal volunteers dosed bilaterally twice daily for 15 days with emedastine ophthalmic solution 0.05%, plasma concentrations of the parent compound were generally below the quantitation limit of the assay (<0.3 ng/mL). Samples in which emedastine was quantifiable ranged from 0.30 to 0.49 ng/mL. The elimination half-life of oral emedastine in plasma is 3-4 hours. Approximately 44% of the oral dose is recovered in the urine over 24 hours with only 3.6% of the dose excreted as parent drug. Two primary metabolites, 5- and 6-hydroxyemedastine, are excreted in the urine as both free and conjugated forms. The 5'-oxoanalogs of 5- and 6-hydroxyemedastine and the N-oxide are also formed as minor metabolites.
- In an environmental study, patients with allergic conjunctivitis were treated with EMADINE® (emedastine difumarate ophthalmic solution) 0.05% for six weeks. The results demonstrated that EMADINE® (emedastine difumarate ophthalmic solution) 0.05% provides relief of the signs and symptoms of allergic conjunctivitis. In conjunctival antigen challenge studies, in which subjects were challenged with antigen both initially and up to four hours after dosing, EMADINE® (emedastine difumarate ophthalmic solution) 0.05% was demonstrated to be significantly more effective than placebo in preventing ocular itching associated with allergic conjunctivitis.
|nonClinToxic=There is limited information regarding Nonclinical Toxicology of Emedastine in the drug label.
|clinicalStudies=There is limited information regarding Clinical Studies of Emedastine in the drug label.
|howSupplied=* EMADINE® (emedastine difumarate ophthalmic solution) 0.05% is supplied as follows:
- 5 mL in opaque, plastic DROP-TAINER® dispenser.
- 5 mL: NDC 0065-0325-05
|storage=* STORAGE: Store at 4° - 30°C (39° - 86°F). |packLabel= |fdaPatientInfo=There is limited information regarding Patient Counseling Information of Emedastine in the drug label.
|alcohol=* Alcohol-Emedastine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|brandNames=* EMADINE®[1]
|drugShortage= }} {{#subobject:
|Label Page=Emedastine |Label Name=E 02.jpg
}}
{{#subobject:
|Label Page=Emedastine |Label Name=E 03.jpg
}}
{{#subobject:
|Label Page=Emedastine |Label Name=DailyMed - EMADINE- emedastine difumarate solution .png
}}