Drospirenone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox| | |||
|IUPAC_name = (6''R'',7''R'',8''R'',9''S'',10''R'',13''S'',14''S'',15''S'',16''S'',17''S'')-<br>1,3',4',6,6a,7,8,9,10,11,12,13,14,15,15a,16-<br>hexadecahydro-10,13-dimethylspiro-<br>[17''H''-dicyclopropa-6,7:15,16]cyclopenta<br>[a]phenanthrene-17,2'(5''H'')-furan]-3,5'(2''H'')-dione) | |||
| image=Drospirenone.svg | |||
| CAS_number=67392-87-4 | |||
| ATC_prefix=G03 | |||
| ATC_suffix=AA12 | |||
| ATC_supplemental= | |||
| PubChem=68873 | |||
| DrugBank= | |||
| C = 24 | H = 30 | O = 3 | |||
| molecular_weight = 366.493 g/mol | |||
| bioavailability= 76% | |||
| metabolism = [[Liver|Hepatic]], minor ([[CYP3A4]]-mediated) | |||
| elimination_half-life= 30 hours | |||
| protein_bound = 97% | |||
| excretion = [[Kidney|Renal]] and fecal | |||
| pregnancy_US = X | |||
| legal_status = | |||
| routes_of_administration= Oral | |||
| | |||
< | |||
: | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
| | |||
}} | }} | ||
{{ | {{SI}} | ||
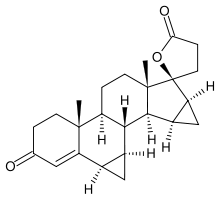
'''Drospirenone''' is a synthetic [[progestin]] that is an [[analog (chemistry)| analog]] to [[spironolactone]]. Its [[molecular weight]] is 366.5 and its [[molecular formula]] is C<sub>24</sub>H<sub>30</sub>O<sub>3</sub>. | |||
}} | |||
| | |||
< | |||
== Properties and uses == | |||
The compound is part of certain [[birth control]] [[oral contraceptive formulations|formulations]]. Drospirenone differs from other synthetic [[progestins]] in that its pharmacological profile in preclinical studies shows it to be closer to the natural [[progesterone]]. As such it has anti-[[mineralocorticoid]] properties, counteracts the [[estrogen]]-stimulated activity of the [[Renin-angiotensin system|renin-angiotensin]]-[[aldosterone]] system, and is not [[androgen]]ic. With its activities similar to [[spironolactone]] it may lead to less water retention and breast tenderness and improved skin appearance (less [[Acne vulgaris|acne]]). | |||
Drospirenone is taken orally with about 76% [[bioavailability]]. It is not bound by [[sex hormone binding globulin]] or [[Corticosteroid binding protein|corticosteroid binding globulin]], but by other serum proteins. Metabolites have not been shown to be biologically active, show up in urine and feces, and are essentially completely excreted within 10 days. | |||
The compound is part of certain newer [[oral contraceptive formulations]]: | |||
* Yasmin® contains 3 mg drospirenone and 30 mcg [[ethinylestradiol]] per tablet. It is indicated for the prevention of pregnancy in women who elect an oral contraceptive. | |||
* Yaz® contains 3 mg drospirenone and 20 mcg ethinylestradiol per tablet and is given for 24/4 days with the same indications. | |||
It has also been formulated in medication to manage [[menopause|menopausal]] symptoms using 0.5 mg drsp and 1 mg [[estradiol]] per day by oral application. This medication was introduced in the USA in 2007 as Angeliq®. | |||
Drospirenone, which can potentially cause [[hyperkalemia]] in high-risk patients, is comparable to a 25mg dose of [[spironolactone]]. | |||
The medication is contraindicated in patients with [[liver disease|hepatic dysfunction]], [[renal insufficiency]], [[adrenal insufficiency]], or in whom the use of oral contraceptives is contraindicated, such as smokers and patients with a history of [[DVT]], [[stroke]], or other blood clots. Because of the anti-mineralocorticoid effects care needs to be exercised when other drugs that may increase potassium levels are taken. Such medications include [[ACE inhibitor]]s, angiotensin-II receptor agonists, [[potassium-sparing diuretic]]s, potassium supplementation, [[heparin]], [[aldosterone antagonist]]s, and [[Non-steroidal anti-inflammatory drug|NSAID]]s. | |||
== See also== | |||
* [[Progestins]] | |||
* [[Combined oral contraceptive pill]] | |||
* [[Oral contraceptive formulations]] | |||
* [[Hormone replacement therapy]] | |||
==References== | |||
* {{cite journal | author = Krattenmacher R | title = Drospirenone: pharmacology and pharmacokinetics of a unique progestogen | journal = Contraception | volume = 62 | issue = 1 | pages = 29-38 | year = 2000 | id = PMID 11024226}} | |||
* [http://www.yasmin-us.com/index.htm Yasmin product information] | |||
* [http://www.angeliq.com/html/index.html Angeliq product information] | |||
==External links== | |||
* [http://www.nlm.nih.gov/medlineplus/druginfo/uspdi/500299.html Medline information] | |||
* [http://www.createforum.com/yasminsideeffec/viewforum.php?f=1&mforum=yasminsideeffec Yasmin Survivors Forum] | |||
[[Category: | {{Sex hormones}} | ||
[[category:Progestagens]] | |||
[[Category:Endocrinology]] | |||
[[de:Drospirenon]] | |||
[[nl:Drospirenon]] | |||
{{WikiDoc Help Menu}} | |||
{{WikiDoc Sources}} | |||
Latest revision as of 16:42, 22 January 2015
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 76% |
| Protein binding | 97% |
| Metabolism | Hepatic, minor (CYP3A4-mediated) |
| Elimination half-life | 30 hours |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C24H30O3 |
| Molar mass | 366.493 g/mol |
|
WikiDoc Resources for Drospirenone |
|
Articles |
|---|
|
Most recent articles on Drospirenone Most cited articles on Drospirenone |
|
Media |
|
Powerpoint slides on Drospirenone |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Drospirenone at Clinical Trials.gov Clinical Trials on Drospirenone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Drospirenone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Drospirenone Discussion groups on Drospirenone Patient Handouts on Drospirenone Directions to Hospitals Treating Drospirenone Risk calculators and risk factors for Drospirenone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Drospirenone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Drospirenone is a synthetic progestin that is an analog to spironolactone. Its molecular weight is 366.5 and its molecular formula is C24H30O3.
Properties and uses
The compound is part of certain birth control formulations. Drospirenone differs from other synthetic progestins in that its pharmacological profile in preclinical studies shows it to be closer to the natural progesterone. As such it has anti-mineralocorticoid properties, counteracts the estrogen-stimulated activity of the renin-angiotensin-aldosterone system, and is not androgenic. With its activities similar to spironolactone it may lead to less water retention and breast tenderness and improved skin appearance (less acne).
Drospirenone is taken orally with about 76% bioavailability. It is not bound by sex hormone binding globulin or corticosteroid binding globulin, but by other serum proteins. Metabolites have not been shown to be biologically active, show up in urine and feces, and are essentially completely excreted within 10 days.
The compound is part of certain newer oral contraceptive formulations:
- Yasmin® contains 3 mg drospirenone and 30 mcg ethinylestradiol per tablet. It is indicated for the prevention of pregnancy in women who elect an oral contraceptive.
- Yaz® contains 3 mg drospirenone and 20 mcg ethinylestradiol per tablet and is given for 24/4 days with the same indications.
It has also been formulated in medication to manage menopausal symptoms using 0.5 mg drsp and 1 mg estradiol per day by oral application. This medication was introduced in the USA in 2007 as Angeliq®.
Drospirenone, which can potentially cause hyperkalemia in high-risk patients, is comparable to a 25mg dose of spironolactone.
The medication is contraindicated in patients with hepatic dysfunction, renal insufficiency, adrenal insufficiency, or in whom the use of oral contraceptives is contraindicated, such as smokers and patients with a history of DVT, stroke, or other blood clots. Because of the anti-mineralocorticoid effects care needs to be exercised when other drugs that may increase potassium levels are taken. Such medications include ACE inhibitors, angiotensin-II receptor agonists, potassium-sparing diuretics, potassium supplementation, heparin, aldosterone antagonists, and NSAIDs.
See also
- Progestins
- Combined oral contraceptive pill
- Oral contraceptive formulations
- Hormone replacement therapy
References
- Krattenmacher R (2000). "Drospirenone: pharmacology and pharmacokinetics of a unique progestogen". Contraception. 62 (1): 29–38. PMID 11024226.
- Yasmin product information
- Angeliq product information
External links
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Progestagens
- Endocrinology