Digoxin overdose
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] ; Associate Editor(s)-in-Chief: Aditya Govindavarjhulla, M.B.B.S. [2]
Overview
Digitalis is a medication prescribed to certain heart patients. Digitalis toxicity is a complication of digitalis therapy, or it may be occur when someone takes more than a large amount of the drug at one time.The most common prescription form of this medication is called digoxin. Digitoxin is another form of digitalis.[1]
Historical Perspective
Digitalis, derived form the foxglove plant, Digitalis purpurea, is mentioned in writings as early as 1250; a Welsh family, known as the Physicians of Myddvai, collected different herbs and digitalis was included in their prescriptions. However, the drug was used erratically until the 18th century, when William Withering, an English physician and botanist, published a monograph describing the clinical effects of an extract of the foxglove plant. Later, in 1785, the indication and the toxicity of digitalis were reported in his book, "An account of the Foxglove and some of its medical uses with practical remarks on dropsy, and other diseases". In Denmark, the leaves of Digitalis purpurea or Digitalis lanata were tested for cardiac glycoside activity. The standardized digitalis powder was used in tinctures, infusions, and tablets. The preparations were included in successive editions of the Danish pharmacopoeia, some of the tinctures already in 1828, i.e. before the standardization of the drug.[2]
Causes
- Single ingestion (Acute intoxication)
- Chronic overmedication
Pathophysiology
Cardiac glycoside (CG) toxicity is in the form of spontaneous Ca2+ waves arising in response to altered function of cardiac ryanodine receptors (RyRs) induced by reactive oxygen species (ROS). It is found that ROS generation oxidized thiol groups on RyRs, increasing their functional activity by increasing sensitivity to Ca2+ in the sarcoplasmic reticulum (SR) . The result is increased frequency of Ca2+ waves which, through sodium–calcium exchange (NCX), depolarize cardiac myocytes and induce triggered arrhythmias during CG toxicity. It is also suggested that this effect of CGs occurs secondary to an interaction with (but not inhibition of) the Na+,K+-ATPase (NKA) that induces intracellular signalling through Src kinase, causing ROS production and opening of the mitochondrial ATP-dependent K+ channel. [3]
It is generally accepted that NKA inhibition by CGs causes both inotropic and toxic actions since the resulting intracellular Na+ accumulation, through NCX, also increases Ca2+. The raised Ca2+ increases contraction (resulting in positive inotropy) but eventually leads to toxicity in the form of Ca2+ waves (and triggered arrhythmias) as SR Ca2+ storage capacity is exceeded and Ca2+ overload develops.
It is evident from few studies that Ca2+waves occurred despite a reduced SR Ca2+ load. Thus, RyR activation occurred at lower SR load, presumably because RyRs were sensitized to luminal Ca2+ after thiol modification. This is an unusual view of Ca2+ wave generation which ordinarily occurs through increased SR Ca2+ load which then increases RyR sensitivity, causing spontaneous release in the form of a wave. There is a compelling evidence that CG induction of ROS with subsequent RyR modification increases functional activity despite a lower SR Ca2+ load. This mechanism may also provide a novel explanation for why toxicity might occur more easily with some CGs than with others; if one agent is more able to induce increased ROS than another, this might explain the differences in toxicity to therapeutic ratio that have been reported among different CGs.
An increase in RyR sensitivity to Ca2+, independent of changes in Na+ and Ca2+, suggests a unique mechanism for CG toxicity in the absence of increased SR load. Clinically, arrhythmias might then be susceptible to treatment with agents that reduce RyR sensitivity, obviating the use of generally ineffective conventional antiarrhythmic agents. Cardiac ROS suppression might also provide a fruitful therapeutic approach to treatment of CG toxicity. Consequently, CG effects on RyRs through a ROS intermediary might provide an entirely new and fresh view of how to deal therapeutically with the extremely common and highly dangerous effects of one of the world's most widely used medications for the treatment of heart disease.
Risk Factors
- People with heart failure who take digoxin are commonly given medications called diuretics, which remove excess fluid from the body. Many diuretics can cause potassium loss. Low levels of potassium in the body increase the risk of digitalis toxicity. Digitalis toxicity may also result in persons who take the drug and who have low levels of magnesium in the body.
- Risks include taking digitalis medications such as digoxin or digitoxin along with medications that interact with digitalis such as quinidine, verapamil, amiodarone, and others.
- Reduced kidney function will cause digitalis to build up in the body rather than be removed normally through urine. Therefore, any disorders that disrupt kidney functioning (including dehydration) make digitalis toxicity more likely.
Natural History, Complications and Prognosis
Prognosis
The outcome varies depending on the extent of toxicity and arrhythmias that develop.
Diagnosis
History and Symptoms
Symptoms of digitalis toxicity
- Confusion
- Irregular pulse
- Loss of appetite
- Nausea, vomiting, diarrhea
- Palpitations
- Visual changes (unusual)
- Blind spots in vision
- Blurred vision
- Changes in color perception
- Halos or rings of light around objects
- Seeing lights or bright spots
Additional symptoms:
- Decreased consciousness
- Decreased urine output
- Difficulty breathing when lying down
- Excessive nighttime urination
- Overall swelling
Laboratory Findings
Blood tests will be done to check:
- BUN and creatinine (which help reveal kidney function)
- Digoxin and digitoxin levels
- Potassium level
- Magnesium level
Electrocardiographic Findings
- AV block may be present including complete AV block and Wenkebach.
- If digoxin levels are extremely high, atrial fibrillation, ventricular tachycardia, and ventricular fibrillation may develop.
It should be noted that the electrocardiographic effects of dig toxicity are increased by hypokalemia.
Shown below is the image of EKG in a patient with digitalis toxicity demonstrating T wave inversions with ST depression in lateral leads

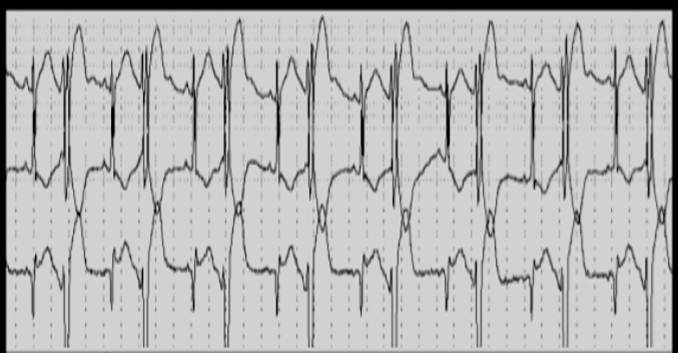
Shown below is the image of EKG in a patient with digitalis toxicity demonstrating increased automaticity
Treatment
Discontinuation
Potassium salts
Dialysis/exchange transfusion/cardiopulmonary bypass
Activated charcoal
Digoxin Immune Fab
Discontinuation
Digoxin should be discontinued until all signs of toxicity are gone. Discontinuation may be all that is necessary if toxic manifestations are not severe and appear only near the expected time for maximum effect of the drug. Return to top
Potassium salts
There has been one reported case of massive overdosage with Nifedipine extended-release tablets. The main effects of ingestion of approximately 4800 mg of Nifedipine extended-release in a young man attempting suicide as a result of cocaine-induced depression was initial dizziness, palpitations, flushing, and nervousness. Within several hours of ingestion, nausea, vomiting, and generalized edema developed. No significant hypotension was apparent at presentation, 18 hours post-ingestion. Electrolyte abnormalities consisted of a mild, transient elevation of serum creatinine, and modest elevations of LDH and CPK, but normal SGOT. Vital signs remained stable, no electrocardiographic abnormalities were noted and renal function returned to normal within 24 to 48 hours with routine supportive measures alone. No prolonged sequelae were observed.Potassium salts may be used, particularly if hypokalemia is present. Potassium chloride in divided oral doses totaling 1 to 1.5 mEq K+ per kilogram (kg) body weight may be given provided renal function is adequate (1 gram of potassium chloride contains 13.4 mEq K+). When correction of the arrhythmia with potassium is urgent and the serum potassium concentration is low or normal, approximately 0.5 mEq/kg of potassium per hour may be given intravenously in 5% dextrose injection. The intravenous solution of potassium should be dilute enough to avoid local irritation; however, especially in infants care must be taken to avoid intravenous fluid overload. ECG monitoring should be performed to watch for any evidence of potassium toxicity (e.g., peaking of T waves) and to observe the effect on the arrhythmia. The infusion may be stopped when the desired effect is achieved.
Note: Potassium should not be used and may be dangerous in heart block due to Digoxin, unless primarily related to supraventricular tachycardia. Return to top
Dialysis/exchange transfusion/cardiopulmonary bypass
Because of its large extravascular volume of distribution, Digoxin is not effectively removed from the body by dialysis, by exchange transfusion, or during cardiopulmonary bypass. Return to top
Activated charcoal
Multiple doses of activated charcoal have been found effective in at least 1 case report, and may be of use while the need for and availability of Digoxin specific antibody fragments are being assessed. In advanced heart block, temporary ventricular pacing may be beneficial. Return to top
Digoxin Immune Fab
Digoxin Immune Fab (Ovine) [DIGIBIND®, DIGIFAB®] may be indicated for the treatment of patients with life-threatening or potentially life-threatening Digoxin toxicity or overdose. Return to top
Adapted from the FDA Package Insert.
References
- ↑ http://www.nlm.nih.gov/medlineplus/ency/article/000165.htm
- ↑ Norn S, Kruse PR. [Cardiac glycosides: From ancient history through Withering's foxglove to endogeneous cardiac glycosides]. Dan Medicinhist Arbog. 2004;: 119-32. - <a href="http://www.ncbi.nlm.nih.gov/pubmed/15685783">Pubmed citation</a>
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/22042543