Difluprednate: Difference between revisions
No edit summary |
m (Protected "Difluprednate": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (3 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{VP}}<!--Overview--> | ||
|aOrAn=a | |||
{{VP}} | |drugClass=topical [[corticosteroid]] | ||
|indicationType=treatment | |||
<!--Overview--> | |indication=[[inflammation]] and [[pain]] associated with ocular surgery and endogenous [[anterior uveitis]] | ||
|adverseReactions=raised [[intraocular pressure]] | |||
|aOrAn= | |||
a | |||
|drugClass= | |||
topical [[corticosteroid]] | |||
|indication= | |||
[[inflammation]] and [[pain]] associated with ocular surgery and endogenous [[anterior uveitis]] | |||
|adverseReactions= | |||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
|blackBoxWarningTitle=Title | |||
|blackBoxWarningTitle= | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | * Content | ||
| Line 39: | Line 16: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult======Ocular Surgery===== | |||
*Instill one drop into the conjunctival sac of the affected eye 4 times daily beginning 24 hours after [[surgery]] and continuing throughout the first 2 weeks of the postoperative period, followed by 2 times daily for a week and then a taper based on the response. | |||
*Instill one drop into the conjunctival sac of the affected eye 4 times daily beginning 24 hours after surgery and continuing throughout the first 2 weeks of the postoperative period, followed by 2 times daily for a week and then a taper based on the response. | |||
=====Endogenous Anterior Uveitis===== | =====Endogenous Anterior Uveitis===== | ||
*Instill one drop into the conjunctival sac of the affected eye 4 times daily for 14 days followed by tapering as clinically indicated. | *Instill one drop into the [[conjunctival]] sac of the affected eye 4 times daily for 14 days followed by tapering as clinically indicated. | ||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
|fdaLIADPed= | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* The use of DUREZOL, as with other ophthalmic [[corticosteroids]], is contraindicated in most active viral diseases of the [[cornea]] and [[conjunctiva]] including epithelial [[herpes simplex]] [[keratitis]] (dendritic [[keratitis]]), [[vaccinia]], and [[varicella]], and also in [[mycobacterial]] infection of the eye and [[fungal]] disease of ocular structures. | |||
|contraindications= | |||
* The use of DUREZOL, as with other ophthalmic corticosteroids, is contraindicated in most active viral diseases of the [[cornea]] and [[conjunctiva]] including epithelial [[herpes simplex]] [[keratitis]] (dendritic [[keratitis]]), [[vaccinia]], and [[varicella]], and also in [[mycobacterial]] infection of the eye and [[fungal]] disease of ocular structures. | |||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=====Precautions==== | |||
|warnings= | |||
====Precautions==== | |||
* IOP Increase | * IOP Increase | ||
:*Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be used with caution in the presence of glaucoma. If this product is used for 10 days or longer, intraocular pressure should be monitored. | :*Prolonged use of [[corticosteroids]] may result in [[glaucoma]] with damage to the [[optic nerve]], defects in visual acuity and fields of vision. Steroids should be used with caution in the presence of [[glaucoma]]. If this product is used for 10 days or longer, [[intraocular pressure]] should be monitored. | ||
*Cataracts | *Cataracts | ||
:*Use of corticosteroids may result in posterior subcapsular cataract formation. | :*Use of [[corticosteroids]] may result in posterior subcapsular [[cataract]] formation. | ||
*Delayed Healing | *Delayed Healing | ||
:*The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation. In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids. The initial prescription and renewal of the medication order beyond 28 days should be made by a physician only after examination of the patient with the aid of magnification such as slit lamp biomicroscopy and, where appropriate, fluorescein staining. | :*The use of [[steroids]] after cataract surgery may delay healing and increase the incidence of bleb formation. In those diseases causing thinning of the [[cornea]] or [[sclera]], perforations have been known to occur with the use of topical [[steroids]]. The initial prescription and renewal of the medication order beyond 28 days should be made by a physician only after examination of the patient with the aid of magnification such as slit lamp biomicroscopy and, where appropriate, fluorescein staining. | ||
*Bacterial Infections | *Bacterial Infections | ||
:*Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, steroids may mask infection or enhance existing infection. If signs and symptoms fail to improve after 2 days, the patient should be re-evaluated. | :*Prolonged use of [[corticosteroids]] may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, steroids may mask infection or enhance existing [[infection]]. If signs and symptoms fail to improve after 2 days, the patient should be re-evaluated. | ||
*Viral Infections | *Viral Infections | ||
:*Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular steroids may prolong the course and may exacerbate the severity of many viral | :*Employment of a [[corticosteroid]] medication in the treatment of patients with a history of [[herpes simplex]] requires great caution. Use of ocular [[steroids]] may prolong the course and may exacerbate the severity of many [[viral infection]]s of the eye (including [[herpes simplex]]). | ||
*Fungal Infections | *Fungal Infections | ||
:*Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local steroid application. Fungus invasion must be considered in any persistent corneal ulceration where a steroid has been used or is in use. Fungal culture should be taken when appropriate. | :*Fungal infections of the [[cornea]] are particularly prone to develop coincidentally with long-term local steroid application. Fungus invasion must be considered in any persistent corneal ulceration where a [[steroid]] has been used or is in use. Fungal culture should be taken when appropriate. | ||
*Topical Ophthalmic Use Only | *Topical Ophthalmic Use Only | ||
:*DUREZOL is not indicated for intraocular administration. | :*DUREZOL is not indicated for [[intraocular]] administration. | ||
*Contact Lens Wear | *Contact Lens Wear | ||
:*DUREZOL should not be instilled while wearing contact lenses. Remove contact lenses prior to instillation of DUREZOL. The preservative in DUREZOL may be absorbed by soft contact lenses. | :*DUREZOL should not be instilled while wearing [[contact lenses]]. Remove contact lenses prior to instillation of DUREZOL. The preservative in DUREZOL may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of DUREZOL. | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=*Ocular Surgery | |||
|clinicalTrials= | :*Ocular adverse reactions occurring in 5-15% of subjects in clinical studies with DUREZOL included [[corneal edema]], ciliary and conjunctival hyperemia, eye pain, [[photophobia]], posterior capsule opacification, anterior chamber cells, anterior chamber flare, [[conjunctival]] edema, and [[blepharitis]]. Other ocular adverse reactions occurring in 1-5% of subjects included reduced [[visual acuity]], punctate [[keratitis]], eye inflammation, and [[iritis]]. Ocular adverse reactions occurring in < 1% of subjects included application site discomfort or irritation, corneal pigmentation and striae, [[episcleritis]], eye [[pruritis]], eyelid irritation and crusting, foreign body sensation, increased [[lacrimation]], [[macular edema]], [[sclera]] hyperemia, and [[uveitis]]. Most of these reactions may have been the consequence of the surgical procedure. | ||
*Ocular Surgery | |||
:*Ocular adverse reactions occurring in 5-15% of subjects in clinical studies with DUREZOL included corneal edema, ciliary and conjunctival hyperemia, eye pain, photophobia, posterior capsule opacification, anterior chamber cells, anterior chamber flare, conjunctival edema, and blepharitis. Other ocular adverse reactions occurring in 1-5% of subjects included reduced visual acuity, punctate keratitis, eye inflammation, and iritis. Ocular adverse reactions occurring in < 1% of subjects included application site discomfort or irritation, corneal pigmentation and striae, episcleritis, eye pruritis, eyelid irritation and crusting, foreign body sensation, increased lacrimation, macular edema, sclera hyperemia, and uveitis. Most of these reactions may have been the consequence of the surgical procedure. | |||
*Endogenous Anterior Uveitis | *Endogenous Anterior Uveitis | ||
:*A total of 200 subjects participated in the clinical trials for endogenous anterior uveitis, of which 106 were exposed to DUREZOL. The most common adverse reactions of those exposed to DUREZOL occurring in 5-10% of subjects included blurred vision, eye irritation, eye pain, headache, increased IOP, iritis, limbal and conjunctival hyperemia, punctate keratitis, and uveitis. Adverse reactions occurring in 2-5% of subjects included anterior chamber flare, corneal edema, dry eye, iridocyclitis, photophobia, and reduced visual acuity. | :*A total of 200 subjects participated in the clinical trials for endogenous anterior [[uveitis]], of which 106 were exposed to DUREZOL. The most common adverse reactions of those exposed to DUREZOL occurring in 5-10% of subjects included [[blurred vision]], eye irritation, eye pain, [[headache]], increased [[IOP]], [[iritis]], limbal and conjunctival hyperemia, punctate [[keratitis]], and [[uveitis]]. Adverse reactions occurring in 2-5% of subjects included anterior chamber flare, corneal edema, [[dry eye]], [[iridocyclitis]], [[photophobia]], and reduced [[visual acuity]]. | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions=<!--Use in Specific Populations--> | |||
|drugInteractions= | |useInPregnancyFDA=* '''Pregnancy Category C''' | ||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category C''' | |||
*Difluprednate has been shown to be embryotoxic (decrease in embryonic body weight and a delay in embryonic ossification) and teratogenic (cleft palate and skeletal anomalies) when administered subcutaneously to rabbits during organogenesis at a dose of 1-10 mcg/kg/day. The no-observed-effect-level (NOEL) for these effects was 1 mcg/kg/day, and 10 mcg/kg/day was considered to be a teratogenic dose that was concurrently found in the toxic dose range for fetuses and pregnant females. Treatment of rats with 10 mcg/kg/day subcutaneously during organogenesis did not result in any reproductive toxicity, nor was it maternally toxic. At 100 mcg/kg/day after subcutaneous administration in rats, there was a decrease in fetal weights and delay in ossification, and effects on weight gain in the pregnant females. It is difficult to extrapolate these doses of difluprednate to maximum daily human doses of DUREZOL, since DUREZOL is administered topically with minimal systemic absorption, and difluprednate blood levels were not measured in the reproductive animal studies. However, since use of difluprednate during human pregnancy has not been evaluated and cannot rule out the possibility of harm, DUREZOL should be used during pregnancy only if the potential benefit justifies the potential risk to the embryo or fetus. | *Difluprednate has been shown to be embryotoxic (decrease in embryonic body weight and a delay in embryonic ossification) and teratogenic (cleft palate and skeletal anomalies) when administered subcutaneously to rabbits during organogenesis at a dose of 1-10 mcg/kg/day. The no-observed-effect-level (NOEL) for these effects was 1 mcg/kg/day, and 10 mcg/kg/day was considered to be a teratogenic dose that was concurrently found in the toxic dose range for fetuses and pregnant females. Treatment of rats with 10 mcg/kg/day subcutaneously during organogenesis did not result in any reproductive toxicity, nor was it maternally toxic. At 100 mcg/kg/day after subcutaneous administration in rats, there was a decrease in fetal weights and delay in ossification, and effects on weight gain in the pregnant females. It is difficult to extrapolate these doses of difluprednate to maximum daily human doses of DUREZOL, since DUREZOL is administered topically with minimal systemic absorption, and difluprednate blood levels were not measured in the reproductive animal studies. However, since use of difluprednate during human pregnancy has not been evaluated and cannot rule out the possibility of harm, DUREZOL should be used during pregnancy only if the potential benefit justifies the potential risk to the embryo or fetus. | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInLaborDelivery= | |useInNursing=*It is not known whether topical ophthalmic administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous [[corticosteroid]] production, or cause other untoward effects. Caution should be exercised when DUREZOL is administered to a nursing woman. | ||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInPed=*DUREZOL was evaluated in a 3-month, multicenter, double-masked trial in 79 pediatric patients (39 DUREZOL; 40 [[prednisolone]] acetate) 0 to 3 years of age for the treatment of inflammation following [[cataract]] surgery. A similar safety profile was observed in pediatric patients comparing DUREZOL to [[prednisolone]] acetate ophthalmic suspension, 1%. | ||
|useInGeri=*No overall differences in safety or effectiveness have been observed between elderly and younger patients. | |||
|useInNursing= | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
*It is not known whether topical ophthalmic administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution should be exercised when DUREZOL is administered to a nursing woman. | |useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | ||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInPed= | |useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
*DUREZOL was evaluated in a 3-month, multicenter, double-masked trial in 79 pediatric patients (39 DUREZOL; 40 prednisolone acetate) 0 to 3 years of age for the treatment of inflammation following cataract surgery. A similar safety profile was observed in pediatric patients comparing DUREZOL to prednisolone acetate ophthalmic suspension, 1%. | |||
|useInGeri= | |||
*No overall differences in safety or effectiveness have been observed between elderly and younger patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=*Topical | |||
|administration= | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
*Topical | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose====Chronic Overdose=== | |||
|overdose= | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | ||
| Line 268: | Line 121: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox={{Drugbox2 | |||
|drugBox= | |||
{{Drugbox2 | |||
| Verifiedfields = changed | | Verifiedfields = changed | ||
| Watchedfields = changed | | Watchedfields = changed | ||
| Line 322: | Line 172: | ||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction=* Corticosteroids inhibit the inflammatory response to a variety of inciting agents and may delay or slow healing. They inhibit [[edema]], [[fibrin]] deposition, capillary dilation, [[leukocyte]] migration, capillary proliferation, [[fibroblast]] proliferation, deposition of collagen, and scar formation associated with inflammation. There is no generally accepted explanation for the mechanism of action of ocular [[corticosteroids]]. However, [[corticosteroids]] are thought to act by the induction of [[phospholipase A2]] inhibitory proteins, collectively called [[lipocortins]]. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as [[prostaglandins]] and [[leukotreines]] by inhibiting the release of their common precursor arachidonic acid. [[Arachidonic acid]] is released from membrane [[phospholipids]] by phospholipase A2. | |||
|mechAction= | |||
* Corticosteroids inhibit the inflammatory response to a variety of inciting agents and may delay or slow healing. They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation. There is no generally accepted explanation for the mechanism of action of ocular corticosteroids. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotreines by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2. | |||
*Difluprednate is structurally similar to other corticosteroids. | *Difluprednate is structurally similar to other corticosteroids. | ||
<!--Structure--> | <!--Structure--> | ||
|structure=* DUREZOL (difluprednate ophthalmic emulsion) 0.05% is a sterile, topical [[anti-inflammatory]] [[corticosteroid]] for ophthalmic use. The chemical name is 6α,9difluoro-11β,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate (CAS number 23674-86-4). Difluprednate is represented by the following structural formula: | |||
|structure= | |||
* DUREZOL (difluprednate ophthalmic emulsion) 0.05% is a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. The chemical name is 6α,9difluoro-11β,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate (CAS number 23674-86-4). Difluprednate is represented by the following structural formula: | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
| Line 342: | Line 186: | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=*Difluprednate undergoes deacetylation in vivo to 6α, 9-difluoroprednisolone 17-butyrate (DFB), an active metabolite of difluprednate. | |||
|PK= | |||
*Difluprednate undergoes deacetylation in vivo to 6α, 9-difluoroprednisolone 17-butyrate (DFB), an active metabolite of difluprednate. | |||
*Clinical pharmacokinetic studies of difluprednate after repeat ocular instillation of 2 drops of difluprednate (0.01% or 0.05%) four times per day for 7 days showed that DFB levels in blood were below the quantification limit (50 ng/mL) at all time points for all subjects, indicating the systemic absorption of difluprednate after ocular instillation of DUREZOL is limited. | *Clinical pharmacokinetic studies of difluprednate after repeat ocular instillation of 2 drops of difluprednate (0.01% or 0.05%) four times per day for 7 days showed that DFB levels in blood were below the quantification limit (50 ng/mL) at all time points for all subjects, indicating the systemic absorption of difluprednate after ocular instillation of DUREZOL is limited. | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic======Carcinogenesis, Mutagenesis, and Impairment of Fertility===== | |||
|nonClinToxic= | |||
=====Carcinogenesis, Mutagenesis, and Impairment of Fertility===== | |||
*Difluprednate was not genotoxic in vitro in the Ames test, and in cultured mammalian cells CHL/IU (a fibroblastic cell line derived from the lungs of newborn female Chinese hamsters). An in vivo micronucleus test of difluprednate in mice was also negative. Treatment of male and female rats with subcutaneous difluprednate up to 10 mcg/kg/day prior to and during mating did not impair fertility in either gender. Long term studies have not been conducted to evaluate the carcinogenic potential of difluprednate. | *Difluprednate was not genotoxic in vitro in the Ames test, and in cultured mammalian cells CHL/IU (a fibroblastic cell line derived from the lungs of newborn female Chinese hamsters). An in vivo micronucleus test of difluprednate in mice was also negative. Treatment of male and female rats with subcutaneous difluprednate up to 10 mcg/kg/day prior to and during mating did not impair fertility in either gender. Long term studies have not been conducted to evaluate the carcinogenic potential of difluprednate. | ||
| Line 368: | Line 203: | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=*Ocular Surgery | |||
:*Clinical efficacy was evaluated in 2 randomized, double-masked, placebo-controlled trials in which subjects with an anterior chamber cell grade ≥ "2" (a cell count of 11 or higher) after [[cataract]] surgery were assigned to DUREZOL or placebo (vehicle) following surgery. One drop of DUREZOL or vehicle was self instilled either 2 times per day or 4 times per day for 14 days, beginning the day after surgery. The presence of complete clearing (a cell count of 0) was assessed 8 and 15 days post-surgery using a slit lamp binocular microscope. In the intent-to-treat analyses of both studies, a significant benefit was seen in the 4 times per day DUREZOL-treated group in ocular [[inflammation]] and reduction of pain when compared with placebo. The consolidated clinical trial results are provided below. | |||
: [[File:{{PAGENAME}}02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: | |||
*Endogenous Anterior Uveitis | *Endogenous Anterior Uveitis | ||
:*Clinical efficacy was evaluated in two randomized, double masked active controlled trials in which patients who presented with endogenous anterior uveitis were treated with either DUREZOL 4 times daily or prednisolone acetate ophthalmic suspension, 1%, 8 times daily for 14 days. Both studies demonstrated that DUREZOL was equally effective as prednisolone acetate ophthalmic suspension, 1% in treating subjects with endogenous anterior uveitis. | :*Clinical efficacy was evaluated in two randomized, double masked active controlled trials in which patients who presented with endogenous anterior [[uveitis]] were treated with either DUREZOL 4 times daily or [[prednisolone]] acetate ophthalmic suspension, 1%, 8 times daily for 14 days. Both studies demonstrated that DUREZOL was equally effective as [[prednisolone]] acetate ophthalmic suspension, 1% in treating subjects with endogenous anterior [[uveitis]]. | ||
: [[File:{{PAGENAME}}03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* DUREZOL (difluprednate ophthalmic emulsion) 0.05% is a sterile, aqueous topical ophthalmic emulsion supplied in an opaque plastic bottle with a controlled drop tip and a pink cap in the following sizes: | |||
|howSupplied= | |||
* DUREZOL (difluprednate ophthalmic emulsion) 0.05% is a sterile, aqueous topical ophthalmic emulsion supplied in an opaque plastic bottle with a controlled drop tip and a pink cap in the following sizes: | |||
:*5 mL in a 8 mL bottle (NDC 0065-9240-07) | :*5 mL in a 8 mL bottle (NDC 0065-9240-07) | ||
:*5 mL in a 7.5 mL bottle (NDC 0065-9240-05) | :*5 mL in a 7.5 mL bottle (NDC 0065-9240-05) | ||
| Line 393: | Line 222: | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo=*Risk of Contamination | |||
|fdaPatientInfo= | |||
*Risk of Contamination | |||
:*This product is sterile when packaged. Advise patients not to allow the dropper tip to touch any surface, as this may contaminate the emulsion. | :*This product is sterile when packaged. Advise patients not to allow the dropper tip to touch any surface, as this may contaminate the emulsion. | ||
:*Use of the same bottle for both eyes is not recommended with topical eye drops that are used in association with [[surgery]]. | |||
*Risk of Secondary Infection | *Risk of Secondary Infection | ||
:*If pain develops, or if redness, itching, or inflammation becomes aggravated, advise patients to consult a physician. | :*If pain develops, or if [[redness]], itching, or [[inflammation]] becomes aggravated, advise patients to consult a physician. | ||
*Contact Lens Wear | *Contact Lens Wear | ||
:*DUREZOL should not be instilled while wearing contact lenses. Advise patients to remove contact lenses prior to instillation of DUREZOL. The preservative in DUREZOL may be absorbed by soft contact lenses. | :*DUREZOL should not be instilled while wearing contact lenses. Advise patients to remove contact lenses prior to instillation of DUREZOL. The preservative in DUREZOL may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of DUREZOL. | ||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* DUREZOL®<ref>{{Cite web | title = DUREZOL durezol emulsion | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d07b65d5-f8e3-4594-a7fb-108218746cec }}</ref> | |||
|brandNames= | |||
* DUREZOL®<ref>{{Cite web | title = DUREZOL durezol emulsion | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d07b65d5-f8e3-4594-a7fb-108218746cec }}</ref> | |||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike=* Durezol® — Durasal®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
|lookAlike= | |||
* Durezol® — Durasal®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
{{PillImage | |||
|fileName=No image.jpg | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}04.png | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}05.png | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | <!--Label Display Image--> | ||
<!--Category--> | <!--Category--> | ||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 19:55, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Difluprednate is a topical corticosteroid that is FDA approved for the treatment of inflammation and pain associated with ocular surgery and endogenous anterior uveitis. Common adverse reactions include raised intraocular pressure.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Ocular Surgery
- Instill one drop into the conjunctival sac of the affected eye 4 times daily beginning 24 hours after surgery and continuing throughout the first 2 weeks of the postoperative period, followed by 2 times daily for a week and then a taper based on the response.
Endogenous Anterior Uveitis
- Instill one drop into the conjunctival sac of the affected eye 4 times daily for 14 days followed by tapering as clinically indicated.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Difluprednate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Difluprednate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Difluprednate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Difluprednate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Difluprednate in pediatric patients.
Contraindications
- The use of DUREZOL, as with other ophthalmic corticosteroids, is contraindicated in most active viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal disease of ocular structures.
Warnings
Precautions
- IOP Increase
- Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be used with caution in the presence of glaucoma. If this product is used for 10 days or longer, intraocular pressure should be monitored.
- Cataracts
- Use of corticosteroids may result in posterior subcapsular cataract formation.
- Delayed Healing
- The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation. In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids. The initial prescription and renewal of the medication order beyond 28 days should be made by a physician only after examination of the patient with the aid of magnification such as slit lamp biomicroscopy and, where appropriate, fluorescein staining.
- Bacterial Infections
- Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, steroids may mask infection or enhance existing infection. If signs and symptoms fail to improve after 2 days, the patient should be re-evaluated.
- Viral Infections
- Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex).
- Fungal Infections
- Topical Ophthalmic Use Only
- DUREZOL is not indicated for intraocular administration.
- Contact Lens Wear
- DUREZOL should not be instilled while wearing contact lenses. Remove contact lenses prior to instillation of DUREZOL. The preservative in DUREZOL may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of DUREZOL.
Adverse Reactions
Clinical Trials Experience
- Ocular Surgery
- Ocular adverse reactions occurring in 5-15% of subjects in clinical studies with DUREZOL included corneal edema, ciliary and conjunctival hyperemia, eye pain, photophobia, posterior capsule opacification, anterior chamber cells, anterior chamber flare, conjunctival edema, and blepharitis. Other ocular adverse reactions occurring in 1-5% of subjects included reduced visual acuity, punctate keratitis, eye inflammation, and iritis. Ocular adverse reactions occurring in < 1% of subjects included application site discomfort or irritation, corneal pigmentation and striae, episcleritis, eye pruritis, eyelid irritation and crusting, foreign body sensation, increased lacrimation, macular edema, sclera hyperemia, and uveitis. Most of these reactions may have been the consequence of the surgical procedure.
- Endogenous Anterior Uveitis
- A total of 200 subjects participated in the clinical trials for endogenous anterior uveitis, of which 106 were exposed to DUREZOL. The most common adverse reactions of those exposed to DUREZOL occurring in 5-10% of subjects included blurred vision, eye irritation, eye pain, headache, increased IOP, iritis, limbal and conjunctival hyperemia, punctate keratitis, and uveitis. Adverse reactions occurring in 2-5% of subjects included anterior chamber flare, corneal edema, dry eye, iridocyclitis, photophobia, and reduced visual acuity.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Difluprednate in the drug label.
Drug Interactions
There is limited information regarding Difluprednate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Difluprednate has been shown to be embryotoxic (decrease in embryonic body weight and a delay in embryonic ossification) and teratogenic (cleft palate and skeletal anomalies) when administered subcutaneously to rabbits during organogenesis at a dose of 1-10 mcg/kg/day. The no-observed-effect-level (NOEL) for these effects was 1 mcg/kg/day, and 10 mcg/kg/day was considered to be a teratogenic dose that was concurrently found in the toxic dose range for fetuses and pregnant females. Treatment of rats with 10 mcg/kg/day subcutaneously during organogenesis did not result in any reproductive toxicity, nor was it maternally toxic. At 100 mcg/kg/day after subcutaneous administration in rats, there was a decrease in fetal weights and delay in ossification, and effects on weight gain in the pregnant females. It is difficult to extrapolate these doses of difluprednate to maximum daily human doses of DUREZOL, since DUREZOL is administered topically with minimal systemic absorption, and difluprednate blood levels were not measured in the reproductive animal studies. However, since use of difluprednate during human pregnancy has not been evaluated and cannot rule out the possibility of harm, DUREZOL should be used during pregnancy only if the potential benefit justifies the potential risk to the embryo or fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Difluprednate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Difluprednate during labor and delivery.
Nursing Mothers
- It is not known whether topical ophthalmic administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution should be exercised when DUREZOL is administered to a nursing woman.
Pediatric Use
- DUREZOL was evaluated in a 3-month, multicenter, double-masked trial in 79 pediatric patients (39 DUREZOL; 40 prednisolone acetate) 0 to 3 years of age for the treatment of inflammation following cataract surgery. A similar safety profile was observed in pediatric patients comparing DUREZOL to prednisolone acetate ophthalmic suspension, 1%.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Difluprednate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Difluprednate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Difluprednate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Difluprednate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Difluprednate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Difluprednate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Difluprednate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Difluprednate in the drug label.
Overdosage
Chronic Overdose
There is limited information regarding Chronic Overdose of Difluprednate in the drug label.
Pharmacology
| |
Difluprednate
| |
| Systematic (IUPAC) name | |
| [(6S,8S,9R,10S,11S,13S,14S,17R)-17-(2-acetyloxyacetyl)-6,9-difluoro-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] butanoate | |
| Identifiers | |
| CAS number | |
| ATC code | D07 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 508.551 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | topical dermatologic |
Mechanism of Action
- Corticosteroids inhibit the inflammatory response to a variety of inciting agents and may delay or slow healing. They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation. There is no generally accepted explanation for the mechanism of action of ocular corticosteroids. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotreines by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
- Difluprednate is structurally similar to other corticosteroids.
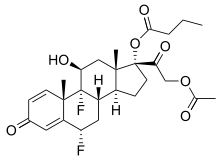
Structure
- DUREZOL (difluprednate ophthalmic emulsion) 0.05% is a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. The chemical name is 6α,9difluoro-11β,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate (CAS number 23674-86-4). Difluprednate is represented by the following structural formula:
- Difluprednate has a molecular weight of 508.56, and the empirical formula is C27H34F2O7.
- Each mL contains: ACTIVE: difluprednate 0.5 mg (0.05%); INACTIVE: boric acid, castor oil, glycerin, polysorbate 80, water for injection, sodium acetate, sodium EDTA, sodium hydroxide (to adjust the pH to 5.2 to 5.8). The emulsion is essentially isotonic with a tonicity of 304 to 411 mOsm/kg. PRESERVATIVE: sorbic acid 0.1%.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Difluprednate in the drug label.
Pharmacokinetics
- Difluprednate undergoes deacetylation in vivo to 6α, 9-difluoroprednisolone 17-butyrate (DFB), an active metabolite of difluprednate.
- Clinical pharmacokinetic studies of difluprednate after repeat ocular instillation of 2 drops of difluprednate (0.01% or 0.05%) four times per day for 7 days showed that DFB levels in blood were below the quantification limit (50 ng/mL) at all time points for all subjects, indicating the systemic absorption of difluprednate after ocular instillation of DUREZOL is limited.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility
- Difluprednate was not genotoxic in vitro in the Ames test, and in cultured mammalian cells CHL/IU (a fibroblastic cell line derived from the lungs of newborn female Chinese hamsters). An in vivo micronucleus test of difluprednate in mice was also negative. Treatment of male and female rats with subcutaneous difluprednate up to 10 mcg/kg/day prior to and during mating did not impair fertility in either gender. Long term studies have not been conducted to evaluate the carcinogenic potential of difluprednate.
Animal Toxicology and/or Pharmacology
- In multiple studies performed in rodents and non-rodents, subchronic and chronic toxicity tests of difluprednate showed systemic effects such as suppression of body weight gain; a decrease in lymphocyte count; atrophy of the lymphatic glands and adrenal gland; and for local effects, thinning of the skin; all of which were due to the pharmacologic action of the molecule and are well known glucocorticosteroid effects. Most, if not all of these effects were reversible after drug withdrawal. The NOEL for the subchronic and chronic toxicity tests were consistent between species and ranged from 1-1.25 mcg/kg/day.
Clinical Studies
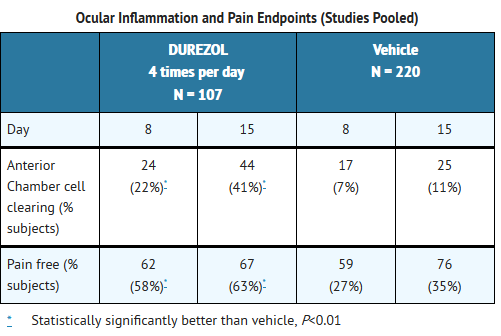
- Ocular Surgery
- Clinical efficacy was evaluated in 2 randomized, double-masked, placebo-controlled trials in which subjects with an anterior chamber cell grade ≥ "2" (a cell count of 11 or higher) after cataract surgery were assigned to DUREZOL or placebo (vehicle) following surgery. One drop of DUREZOL or vehicle was self instilled either 2 times per day or 4 times per day for 14 days, beginning the day after surgery. The presence of complete clearing (a cell count of 0) was assessed 8 and 15 days post-surgery using a slit lamp binocular microscope. In the intent-to-treat analyses of both studies, a significant benefit was seen in the 4 times per day DUREZOL-treated group in ocular inflammation and reduction of pain when compared with placebo. The consolidated clinical trial results are provided below.
- Endogenous Anterior Uveitis
- Clinical efficacy was evaluated in two randomized, double masked active controlled trials in which patients who presented with endogenous anterior uveitis were treated with either DUREZOL 4 times daily or prednisolone acetate ophthalmic suspension, 1%, 8 times daily for 14 days. Both studies demonstrated that DUREZOL was equally effective as prednisolone acetate ophthalmic suspension, 1% in treating subjects with endogenous anterior uveitis.
How Supplied
- DUREZOL (difluprednate ophthalmic emulsion) 0.05% is a sterile, aqueous topical ophthalmic emulsion supplied in an opaque plastic bottle with a controlled drop tip and a pink cap in the following sizes:
- 5 mL in a 8 mL bottle (NDC 0065-9240-07)
- 5 mL in a 7.5 mL bottle (NDC 0065-9240-05)
- Storage and Handling
- Store at 15-25°C (59-77°F). Do not freeze. Protect from light. When not in use, keep the bottles in the protective carton.
Storage
There is limited information regarding Difluprednate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Difluprednate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Difluprednate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Risk of Contamination
- This product is sterile when packaged. Advise patients not to allow the dropper tip to touch any surface, as this may contaminate the emulsion.
- Use of the same bottle for both eyes is not recommended with topical eye drops that are used in association with surgery.
- Risk of Secondary Infection
- If pain develops, or if redness, itching, or inflammation becomes aggravated, advise patients to consult a physician.
- Contact Lens Wear
- DUREZOL should not be instilled while wearing contact lenses. Advise patients to remove contact lenses prior to instillation of DUREZOL. The preservative in DUREZOL may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of DUREZOL.
Precautions with Alcohol
- Alcohol-Difluprednate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- DUREZOL®[1]
Look-Alike Drug Names
- Durezol® — Durasal®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "DUREZOL durezol emulsion".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Difluprednate
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Difluprednate |Label Name=Difluprednate04.png
}}
{{#subobject:
|Label Page=Difluprednate |Label Name=Difluprednate05.png
}}