Cervical cancer screening
|
Cervical cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Cervical cancer screening On the Web |
|
American Roentgen Ray Society Images of Cervical cancer screening |
|
Risk calculators and risk factors for Cervical cancer screening |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Assistant Editor-in-Chief: Monalisa Dmello, M.B,B.S., M.D. [2]
Overview
Pap smear is a medical screening method, invented by Georgios Papanikolaou, primarily designed to detect premalignant and malignant processes in the ectocervix. It may also detect infections and abnormalities in the endocervix and endometrium.
Procedure
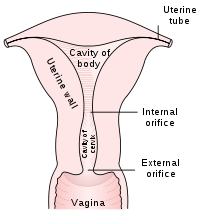
The pre-cancerous changes (called dysplasias or cervical or endocervical intraepithelial neoplasia) are usually caused by sexually transmitted human papillomaviruses (HPVs). The test aims to detect and prevent the progression of HPV-induced cervical cancer and other abnormalities in the female genital tract by sampling cells from the outer opening of the cervix (Latin for "neck") of the uterus and the endocervix. It is generally recommended that sexually active females seek Pap smear testing annually, although guidelines may vary from country to country. If results are abnormal, and depending on the nature of the abnormality, the test may need to be repeated in three to twelve months. If the abnormality requires closer scrutiny, the patient may be referred for detailed inspection of the cervix by colposcopy. The patient may also be referred for HPV DNA testing, which can serve as an adjunct (or even as an alternative) to Pap testing.
About 5% to 7% of pap smears produce abnormal results, such as dysplasia, possibly indicating a pre-cancerous condition. Although many low grade cervical dysplasias spontaneously regress without ever leading to cervical cancer, dysplasia can serve as an indication that increased vigilance is needed. Endocervical and endometrial abnormalities can also be detected, as can a number of infectious processes, including yeast and Trichomonas vaginalis. A small proportion of abnormalities are reported as of "uncertain significance".
Technical Aspects
Samples are collected from the outer opening or os of the cervix using an Aylesbury spatula or (more frequently with the advent of liquid-based cytology) a plastic-fronded broom. The cells are placed on a glass slide and checked for abnormalities in the laboratory.
The sample is stained using the Papanicolaou technique, in which tinctorial dyes and acids are selectively retained by cells. Unstained cells can not be visualized with light microscopy. The stains chosen by Papanicolau were selected to highlight cytoplasmic keratinization, which actually has almost nothing to do with the nuclear features used to make diagnoses now.
The sample is then screened by a specially trained and qualified cytotechnologist using a light microscope. The terminology for who screens the sample varies according the country; in the UK, the personnel are known as Cytoscreeners, Biomedical scientists (BMS), Advanced Practitioners and Pathologists. The latter two take responsibility for reporting the abnormal sample which may require further investigation.
Studies of the accuracy of conventional cytology report:
- Sensitivity 72%[1]
- Specificity 94%[1]
Liquid Based Monolayer Cytology
The techniques based around placing the sample into a vial containing a liquid medium which preserves the cells have been increasingly used. The media are primarily ethanol based. Two of the types are Sure-Path (TriPath Imaging) and Thin-Prep (Cytyc Corp). Once placed into the vial, the sample is processed at the laboratory into a cell thin-layer, stained, and examined by light microscopy. The liquid sample has the advantage of being suitable for low and high risk HPV testing and reduced unsatisfactory specimens from 4.1% to 2.6%.[2] Proper sample acquisition is crucial to the accuracy of the test; clearly, a cell that is not in the sample cannot be evaluated.
Studies of the accuracy of liquid based monolayer cytology report:
- Sensitivity 61%[3] to 66%[1]
- Specificity 82%[3] to 91%[1]
Some[2], but not all studies[1][3], report increased sensitivity from the liquid based smears.
Results



The Bethesda system (TBS) is a system for reporting cervical or vaginal cytologic diagnoses,[4] used for reporting Pap smear results.
- Types of results
- Abnormal results include
- Atypical squamous cells
- Atypical squamous cells of undetermined significance (ASC-US)
- Atypical squamous cells – cannot exclude HSIL (ASC-H)
- Low grade squamous intraepithelial lesion (LGSIL or LSIL)
- High grade squamous intraepithelial lesion (HGSIL or HSIL)
- Squamous cell carcinoma
- Atypical Glandular Cells not otherwise specified (AGC-NOS)
- Atypical Glandular Cells, suspicious for AIS or cancer (AGC-neoplastic)
- Adenocarcinoma in situ (AIS)
Effectiveness
The Pap test, when combined with a regular program of screening and appropriate follow-up, can reduce cervical cancer deaths by up to 80%.[5] Failure of prevention of cancer by the Pap test can occur for many reasons, including not getting regular screening, lack of appropriate follow up of abnormal results, and sampling and interpretation errors.[6] In the US, over half of all invasive cancers occur in women that have never had a Pap smear; an additional 10 to 20% of cancers occur in women that have not had a Pap smear in the preceding five years. About one-quarter of US cervical cancers were in women that had an abnormal Pap smear, but did not get appropriate follow-up (woman did not return for care, or clinician did not perform recommended tests or treatment).
Adenocarcinoma of the cervix has not been shown to be prevented by Pap tests.[6] In the UK, which has a Pap smear screening program, Adenocarcinoma accounts for about 15% of all cervical cancers[7]
Screening With the Pap Test: Harms
Based on solid evidence, regular screening with the Pap test leads to additional diagnostic procedures (e.g., colposcopy) and treatment for low-grade squamous intraepithelial lesions (LSIL), with long-term consequences for fertility and pregnancy. These harms are greatest for younger women, who have a higher prevalence of LSIL, lesions that often regress without treatment. Harms are also increased in younger women because they have a higher rate of false-positive results. Magnitude of Effect: Additional diagnostic procedures were performed in 50% of women undergoing regular Pap testing. Approximately 5% were treated for LSIL. The number with impaired fertility and pregnancy complications is unknown.
Human Papillomavirus Testing
The presence of HPV indicates that the person has been infected, the majority of women who get infected will successfully clear the infection within 18 months. It is those who have an infection of prolonged duration with high risk types[8] (e.g. types 16,18,31,45) that are more likely to develop Cervical Intraepithelial Neoplasia due to the effects that HPV has on DNA.
Studies of the accuracy of HPV testing report:
- Sensitivity 88% to 91% (for detecting CIN 3 or higher)[3] to 97% (for detecting CIN2+)[9]
- Specificity 73% to 79% (for detecting CIN 3 or higher)[3] to 93% (for detecting CIN 3 or higher)[9]
By adding the more sensitive HPV Test, the specificity may decline. However, the drop in specificity is not definite. [10] If the specificity does decline, this results in increased numbers of false positive tests and many women who did not have disease having colposcopy[11] and treatment. A worthwhile screening test requires a balance between the sensitivity and specificity to ensure that those having a disease are correctly identified as having it and equally importantly those not identifying those without the disease as having it. Due to the liquid based pap smears having a false negative rate of 15-35%, the American College of Obstetricians and Gynecologists and American Society for Colposcopy and Cervical Pathology[12] have recommended the use of HPV testing in addition to the pap smear in all women over the age of 30.
Screening With the Human Papillomavirus (HPV) DNA Test: Benefits
Based on solid evidence, screening with HPV DNA or HPV RNA detects high-grade cervical dysplasia, a precursor lesion for cervical cancer. Additional clinical trials show that HPV testing is superior to other cervical cancer screening strategies. In April 2014, the U.S. Food and Drug Administration approved an HPV DNA test that can be used alone for the primary screening of cervical cancer risk in women aged 25 years and older. Magnitude of Effect: In one prospective, clustered, randomized trial, HPV testing was superior to other strategies for preventing cervical cancer mortality. [13] Regarding the role of HPV testing, randomized controlled trials have compared HPV to colposcopy. HPV testing appears as sensitive as immediate colposcopy while reducing the number of colposcopies needed.[14] Randomized controlled trial have suggested that HPV testing could follow abnormal cytology[3] or could precede cervical cytology examination.[9]
Screening With the HPV DNA Test: Harms
Based on solid evidence, HPV testing identifies numerous infections that will not lead to cervical dysplasia or cervical cancer. This is especially true in women younger than 30 years, in whom rates of HPV infection may be higher. In one study, 86.7 % of women with a positive HPV test did not develop cervical cancer or related premalignant disease after more than a decade of follow up.[15]
Screening With the Pap Test and the HPV DNA Test (Cotesting): Benefits
Based on solid evidence, screening every 5 years with the Pap test and the HPV DNA test (cotesting) in women 30 years and older is more sensitive in detecting cervical abnormalities, compared with the Pap test alone. Screening with the Pap test and HPV DNA test reduces the incidence of cervical cancer.HPV-based screening provides 60% to 70% greater protection against invasive cervical carcinoma, compared with cytology.[16]
Screening With the Pap Test and the HPV DNA Test (Cotesting): Harms
Based on solid evidence, HPV and Pap cotesting is associated with more false-positives than the Pap test alone. Abnormal test results can lead to more frequent testing and invasive diagnostic procedures.The percentage of U.S. women undergoing cotesting who will have a normal cytology test result and a positive HPV test result (and who will therefore require additional testing) ranges from 11% among women aged 30 to 34 years to 2.6% among women aged 60 to 65 years.[17]
Screening Women Without a Cervix
Based on solid evidence, screening is not helpful in women who do not have a cervix as a result of a hysterectomy for a benign condition. Among women without cervices, fewer than 1 per 1,000 had abnormal Pap test results.[18]
Automated Analysis
In the last decade there have been successful attempts to develop automated, computer image analysis systems for screening.[19] Automation may improve sensitivity and reduce unsatisfactory specimens.[20] One of these has been FDA approved and functions in high volume reference laboratories, with human oversight.
Screening Guidelines
| American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology | U.S. Preventive Services Task Force (USPSTF) | American College of Obstetricians and Gynecologists (ACOG) | ||
|---|---|---|---|---|
| When to start screening | Age 21. Women aged <21 years should not be screened regardless of the age of sexual initiation or other risk factors | Age 21. (A recommendation) Recommend against screening women aged <21 years (D recommendation) | Age 21 regardless of the age of onset of sexual activity. Women aged <21 years should not be screened regardless of age at sexual initiation and other behavior-related risk factors (Level A evidence) | |
| Statement about annual screening | Women of any age should not be screened annually by any screening method | Individuals and clinicians can use the annual Pap test screening visit as an opportunity to discuss other health problems and preventive measures. Individuals, clinicians, and health systems should seek effective ways to facilitate the receipt of recommended preventive services at intervals that are beneficial to the patient. Efforts also should be made to ensure that individuals are able to seek care for additional health concerns as they present | In women aged 30–65 years, annual cervical cancer screening should not be performed. (Level A evidence) Patients should be counseled that annual well-woman visits are recommended even if cervical cancer screening is not performed at each visit | |
| Screening method and intervals | ||||
| Cytology (conventional or liquid based) | 21–29 years | Every 3 years | Every 3 years (A recommendation) | Every 3 years (Level A evidence) |
| 30–65 years | Every 3 years | Every 3 years (A recommendation) | Every 3 years (Level A evidence) | |
| HPV co-test (cytology + HPV test administered together) | 21–29 years | HPV co-testing should not be used for women aged <30 years | Recommend against HPV co-testing in women aged <30 years (D recommendation) | HPV co-testing should not be performed in women aged <30 years. (Level A evidence ) |
| 30–65 years | Every 5 years; this is the preferred method. | For women who want to extend their screening interval, HPV co-testing every 5 years is an option (A recommendation) | Every 5 years; this is the preferred method (Level A evidence) | |
| Primary hrHPV f testing (as an alternative to cotesting or g cytology alone) | For women aged 30–65 years, screening by HPV testing alone is not recommended in most clinical settings | Recommend against screening for cervical cancer with HPV testing (alone or in combination with cytology) in women aged <30 years (D recommendation) | Not addressed | |
| When to stop screening | Aged >65 years with adequate negative prior screening* and no history of CIN2 or higher within the last 20 years | Aged >65 years with adequate screening history* and are not otherwise at high risk for cervical cancer (D recommendation) | Aged >65 years with adequate negative prior screening* results and no history of CIN 2 or higher (Level A evidence) | |
| When to screen after age 65 years | When to screen after age 65 years Aged >65 years with a history of CIN2 CIN2, CIN3, or adenocarcinoma in situ, routine screeningk should continue for at least 20 years | Women aged >65 years who have never been screened, do not meet the criteria for adequate prior screening, or for whom the adequacy of prior screening cannot be accurately accessed or documented.l Routine screeningk should continue for at least 20 years after spontaneous regression or appropriate management of a high-grade precancerous lesion, even if this extends screening past age 65 years. Certain considerations may support screening in women aged > 65 years who are otherwise considered high risk (such as women with a highgrade precancerous lesion or cervical cancer, women with in utero exposure to diethylstilbestrol, or women who are immunocompromised) | Women aged >65 years with a history of CIN2, CIN3, or AIS should continue routine agebased screeningk for at least 20 years (Level B evidence) | |
| Screening post-hysterectomy | Women who have had a total hysterectomy (removal of the uterus and cervix) should stop screening.Women who have had a supra-cervical hysterectomy (cervix intact) should continue screening according to guidelines | Recommend against screening in women who have had a hysterectomy (removal of the cervix) (D recommendation) | Women who have had a hysterectomy (removal of the cervix) should stop screening and not restart for any reason, (Level A evidence) | |
| The need for a bimanual pelvic exam | Not addressed in 2012 guidelines but was addressed in 2002 ACS guidelines | Addressed in USPSTF ovarian cancer screening recommendations (draft) | Addressed in 2012 well-woman visit recommendations.Aged <21 years, no evidence supports the routine internal examination of the healthy, asymptomatic patient. An “external-only” genital examination is acceptable. Aged ≥21 years, no evidence supports or refutes the annual pelvic examination or speculum and bimanual examination. The decision whether or not to perform a complete pelvic examination should be a shared decision after a discussion between the patient and her health care provider. Annual examination of the external genitalia should continue | |
| Screening among those immunized against HPV 16/18 | Women at any age with a history of HPV vaccination should be screened according to the age specific recommendations for the general population | The possibility that vaccination might reduce the need for screening with cytology alone or in combination with HPV testing is not established. Given these uncertainties, women who have been vaccinated should continue to be screened | Women who have received the HPV vaccine should be screened according to the same guidelines as women who have not been vaccinated (Level C evidence) | |
HPV = human papillomavirus; CIN = cervical intraepithelial neoplasia; AIS=adenocarcinoma in situ; hrHPV = high-risk HPV.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Coste J, Cochand-Priollet B, de Cremoux P; et al. (2003). "Cross sectional study of conventional cervical smear, monolayer cytology, and human papillomavirus DNA testing for cervical cancer screening". BMJ. 326 (7392): 733. doi:10.1136/bmj.326.7392.733. PMID 12676841. ACP Journal Club
- ↑ 2.0 2.1 Ronco G, Cuzick J, Pierotti P; et al. (2007). "Accuracy of liquid based versus conventional cytology: overall results of new technologies for cervical cancer screening randomised controlled trial". doi:10.1136/bmj.39196.740995.BE. PMID 17517761.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Kulasingam SL, Hughes JP, Kiviat NB; et al. (2002). "Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral". JAMA. 288 (14): 1749–57. PMID 12365959.
- ↑ Apgar BS, Zoschnick L, Wright TC (2003). "The 2001 Bethesda System terminology". Am Fam Physician. 68 (10): 1992–8. PMID 14655809.
- ↑ M. Arbyn; et al. (2010). "European Guidelines for Quality Assurance in Cervical Cancer Screening. Second Edition—Summary Document". Annals of Oncology. 21 (3): 448–458. doi:10.1093/annonc/mdp471. PMC 2826099. PMID 20176693.
- ↑ 6.0 6.1 DeMay, M. (2007). Practical principles of cytopathology. Revised edition. Chicago, IL: American Society for Clinical Pathology Press. ISBN 978-0-89189-549-7.
- ↑ "Cancer Research UK website". Retrieved 2009-01-03.
- ↑ Cuschieri KS, Cubie HA, Whitley MW; et al. (2005). "Persistent high risk HPV infection associated with development of cervical neoplasia in a prospective population study". J. Clin. Pathol. 58 (9): 946–50. doi:10.1136/jcp.2004.022863. PMID 16126875.
- ↑ 9.0 9.1 9.2 Cuzick J, Szarewski A, Cubie H; et al. (2003). "Management of women who test positive for high-risk types of human papillomavirus: the HART study". Lancet. 362 (9399): 1871–6. PMID 14667741.
- ↑ Arbyn M, Buntinx F, Van Ranst M, Paraskevaidis E, Martin-Hirsch P, Dillner J (2004). "Virologic versus cytologic triage of women with equivocal Pap smears: a meta-analysis of the accuracy to detect high-grade intraepithelial neoplasia". J. Natl. Cancer Inst. 96 (4): 280–93. PMID 14970277.
- ↑ http://screening.iarc.fr/colpochap.php?lang=1&chap=4
- ↑ Wright TC, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ (2002). "2001 Consensus Guidelines for the management of women with cervical cytological abnormalities". JAMA. 287 (16): 2120–9. PMID 11966387.
- ↑ http://www.cancer.gov/types/cervical/hp/cervical-screening-pdq#link/_133_toc
- ↑ ASCUS-LSIL Traige Study (ALTS) Group. (2003). "Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance". Am. J. Obstet. Gynecol. 188 (6): 1383–92. PMID 12824967.
- ↑ http://www.cancer.gov/types/cervical/hp/cervical-screening-pdq#link/_133_toc
- ↑ http://www.cancer.gov/types/cervical/hp/cervical-screening-pdq#link/_133_toc
- ↑ http://www.cancer.gov/types/cervical/hp/cervical-screening-pdq#link/_133_toc
- ↑ http://www.cancer.gov/types/cervical/hp/cervical-screening-pdq#link/_133_toc
- ↑ Biscotti CV, Dawson AE, Dziura B; et al. (2005). "Assisted primary screening using the automated ThinPrep Imaging System". Am. J. Clin. Pathol. 123 (2): 281–7. PMID 15842055.
- ↑ Davey E, d'Assuncao J, Irwig L; et al. (2007). "Accuracy of reading liquid based cytology slides using the ThinPrep Imager compared with conventional cytology: prospective study". doi:10.1136/bmj.39219.645475.55. PMID 17604301.