Cefacetrile: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
No edit summary |
||
| Line 1: | Line 1: | ||
<div style="float: right;"> | |||
[[File:Cefacetrile.png|thumb|none|400px|This image is provided by the National Library of Medicine.]]</div> | |||
__NOTOC__ | |||
| | |||
| | |||
| | |||
| | |||
{{SI}} | {{SI}} | ||
{{CMG}} | |||
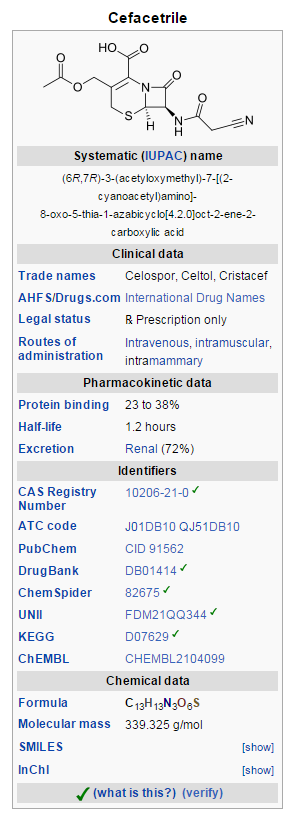
'''Cefacetrile''' ([[International Nonproprietary Name|INN]], also spelled cephacetrile) is a [[Broad-spectrum antibiotic|broad-spectrum]] first generation [[cephalosporin]] [[antibiotic]] effective in [[ | ==Overview== | ||
'''Cefacetrile''' ([[International Nonproprietary Name|INN]], also spelled cephacetrile) is a [[Broad-spectrum antibiotic|broad-spectrum]] first generation [[cephalosporin]] [[antibiotic]] effective in [[gram-positive]] and [[gram-negative]] bacterial infections. It is a [[bacteriostatic]] antibiotic.<ref>{{cite web|url=http://www.emea.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500011465.pdf|publisher=[[European Medicines Agency]], Committee for Veterinary Medicinal Products|title=Cefacetrile Summary Report|year=1998}}</ref><ref name="AC">{{cite book|title=Austria-Codex|editor=Haberfeld, H|publisher=Österreichischer Apothekerverlag|location=Vienna|year=2007|edition=2007/2008|isbn=3-85200-183-8|language=German}}</ref> Cefacetrile is marketed under the trade names '''Celospor''', '''Celtol''', and '''Cristacef''',<ref>{{cite pmid|7206219}}</ref> and as '''Vetimast''' for the treatment of [[mammary]] infections in lactating cows.<ref name="AC" /> | |||
==References== | |||
{{Reflist|2}} | |||
{{CephalosporinAntiBiotics}} | {{CephalosporinAntiBiotics}} | ||
[[Category:Cephalosporin antibiotics]] | [[Category:Cephalosporin antibiotics]] | ||
[[Category:Carboxylate esters]] | |||
[[Category:Drug]] | |||
[[ | |||
Revision as of 15:47, 7 April 2015
|
WikiDoc Resources for Cefacetrile |
|
Articles |
|---|
|
Most recent articles on Cefacetrile Most cited articles on Cefacetrile |
|
Media |
|
Powerpoint slides on Cefacetrile |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Cefacetrile at Clinical Trials.gov Clinical Trials on Cefacetrile at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cefacetrile
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Cefacetrile Discussion groups on Cefacetrile Patient Handouts on Cefacetrile Directions to Hospitals Treating Cefacetrile Risk calculators and risk factors for Cefacetrile
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cefacetrile |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Cefacetrile (INN, also spelled cephacetrile) is a broad-spectrum first generation cephalosporin antibiotic effective in gram-positive and gram-negative bacterial infections. It is a bacteriostatic antibiotic.[1][2] Cefacetrile is marketed under the trade names Celospor, Celtol, and Cristacef,[3] and as Vetimast for the treatment of mammary infections in lactating cows.[2]
References
- ↑ "Cefacetrile Summary Report" (PDF). European Medicines Agency, Committee for Veterinary Medicinal Products. 1998.
- ↑ 2.0 2.1 Haberfeld, H, ed. (2007). Austria-Codex (in German) (2007/2008 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 3-85200-183-8.
- ↑ PMID 7206219 (PMID 7206219)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand