Aromatase
| Cytochrome P450, family 19, subfamily A, polypeptide 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||
| Symbols | CYP19A1 ; ARO; ARO1; CPV1; CYAR; CYP19; MGC104309; P-450AROM | ||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 30955 | ||||||||||
| |||||||||||
| RNA expression pattern | |||||||||||
 | |||||||||||
| More reference expression data | |||||||||||
| Orthologs | |||||||||||
| Template:GNF Ortholog box | |||||||||||
| Species | Human | Mouse | |||||||||
| Entrez | n/a | n/a | |||||||||
| Ensembl | n/a | n/a | |||||||||
| UniProt | n/a | n/a | |||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||
| RefSeq (protein) | n/a | n/a | |||||||||
| Location (UCSC) | n/a | n/a | |||||||||
| PubMed search | n/a | n/a | |||||||||
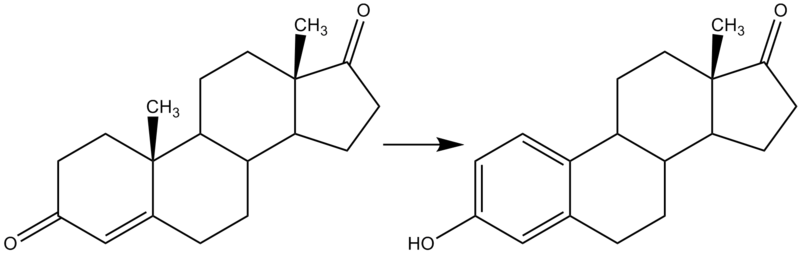
Aromatase is an enzyme of the cytochrome P450 superfamily (EC 1.14.14.1), whose function is to aromatize androgens (that is, to selectively increase their aromaticity), producing estrogens. As such, it is an important factor in sexual development.
This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. This protein localizes to the endoplasmic reticulum and catalyzes the last steps of estrogen biosynthesis, three successive hydroxylations of the A ring of androgens. Mutations in this gene can result in either increased or decreased aromatase activity; the associated phenotypes suggest that estrogen functions both as a sex steroid hormone and in growth or differentiation. The gene expresses two transcript variants.[1]

Genomics
In humans, the gene CYP19, located on chromosome 15q21.1, encodes the aromatase enzyme. CYP19 is present in an early-diverging chordate, the cephalochordate amphioxus (the Florida lancelet, Branchiostoma floridae), but not in the earlier diverging tunicate Ciona intestinalis. Thus the aromatase gene evolved early in chordate evolution and does not appear to be present in non-chordate invertebrates (e.g. insects, molluscs, echinoderms, sponges, corals). However, estrogens may be synthesized in some of these organisms, via unknown pathways.
Cellular and tissue location
The enzyme is located in the endoplasmic reticulum of the cell and its activity is regulated by tissue specific promoters that are in turn controlled by hormones, cytokines, and other factors. The principal action of the enzyme transforms androstenedione to estrone and testosterone to estradiol. The aromatase enzyme can be found in many tissues including gonads, brain, adipose tissue, placenta, blood vessels, skin, bone, endometrium as well as in tissue of endometriosis, uterine fibroids, breast cancer, and endometrial cancer.
Activity
Factors known to increase aromatase activity include age, obesity, insulin, gonadotropins, and alcohol. Aromatase activity is decreased by prolactin, AMH, and smoking. Aromatase activity appears to be enhanced in certain estrogen-dependent local tissue next to breast cancer, endometrial cancer, endometriosis, and uterine fibroids.
Disorders
Aromatase excess syndrome
A number of investigators have reported on a rather rare syndrome of excess aromatase activity. In boys it can lead to gynecomastia and in girls to precocious puberty and gigantomastia. In both sexes, early epiphyseal closure leads to shortness.
Aromatase deficiency syndrome
This syndrome is due to a mutation of gene CYP19 and inherited in an autosomal recessive way. Accumulations of androgens during pregnancy may lead to virilization of a female at birth (males are not affected). Females will have primary amenorrhea. Individuals of both sexes will be tall as lack of estrogen does not bring the epiphyseal lines to closure.
Aromatase inhibitors
The inhibition of the enzyme leads to profound hypoestrogenism (low estrogen levels). Thus aromatase inhibitors have become useful in the management of patients with breast cancer whose lesion was found to be estrogen receptor positive.
References
Further reading
- Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril 2006;85:1307-18. PMID 16647373
- Chen S (2004). "Aromatase and breast cancer". Front. Biosci. 3: d922–33. PMID 9696881.
- Strobel HW, Thompson CM, Antonovic L (2001). "Cytochromes P450 in brain: function and significance". Curr. Drug Metab. 2 (2): 199–214. PMID 11469726.
- Simpson ER, Clyne C, Rubin G; et al. (2002). "Aromatase--a brief overview". Annu. Rev. Physiol. 64: 93–127. doi:10.1146/annurev.physiol.64.081601.142703. PMID 11826265.
- Bulun SE, Yang S, Fang Z; et al. (2002). "Role of aromatase in endometrial disease". J. Steroid Biochem. Mol. Biol. 79 (1–5): 19–25. PMID 11850203.
- Balthazart J, Baillien M, Ball GF (2002). "Phosphorylation processes mediate rapid changes of brain aromatase activity". J. Steroid Biochem. Mol. Biol. 79 (1–5): 261–77. PMID 11850233.
- Richards JA, Petrel TA, Brueggemeier RW (2002). "Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells". J. Steroid Biochem. Mol. Biol. 80 (2): 203–12. PMID 11897504.
- Balthazart J, Baillien M, Ball GF (2002). "Interactions between aromatase (estrogen synthase) and dopamine in the control of male sexual behavior in quail". Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 132 (1): 37–55. PMID 11997208.
- Meinhardt U, Mullis PE (2002). "The aromatase cytochrome P-450 and its clinical impact". Horm. Res. 57 (5–6): 145–52. PMID 12053085.
- Carreau S, Bourguiba S, Lambard S; et al. (2003). "Reproductive system: aromatase and estrogens". Mol. Cell. Endocrinol. 193 (1–2): 137–43. PMID 12161013.
- Meinhardt U, Mullis PE (2003). "The essential role of the aromatase/p450arom". Semin. Reprod. Med. 20 (3): 277–84. doi:10.1055/s-2002-35374. PMID 12428207.
- Carreau S, Bourguiba S, Lambard S, Galeraud-Denis I (2003). "[Testicular aromatase]". J. Soc. Biol. 196 (3): 241–4. PMID 12462076.
- Carani C, Fabbi M, Zirilli L, Sgarbi I (2003). "[Estrogen resistance and aromatase deficiency in humans]". J. Soc. Biol. 196 (3): 245–8. PMID 12462077.
- Kragie L (2003). "Aromatase in primate pregnancy: a review". Endocr. Res. 28 (3): 121–8. PMID 12489562.
- Simpson ER (2004). "Biology of aromatase in the mammary gland". Journal of mammary gland biology and neoplasia. 5 (3): 251–8. PMID 14973387.
- Bulun SE, Takayama K, Suzuki T; et al. (2004). "Organization of the human aromatase p450 (CYP19) gene". Semin. Reprod. Med. 22 (1): 5–9. doi:10.1055/s-2004-823022. PMID 15083376.
- Simpson ER (2004). "Aromatase: biologic relevance of tissue-specific expression". Semin. Reprod. Med. 22 (1): 11–23. doi:10.1055/s-2004-823023. PMID 15083377.
- Bulun SE, Fang Z, Imir G; et al. (2004). "Aromatase and endometriosis". Semin. Reprod. Med. 22 (1): 45–50. doi:10.1055/s-2004-823026. PMID 15083380.
- Shozu M, Murakami K, Inoue M (2004). "Aromatase and leiomyoma of the uterus". Semin. Reprod. Med. 22 (1): 51–60. doi:10.1055/s-2004-823027. PMID 15083381.
- Chen S, Ye J, Kijima I; et al. (2005). "Positive and negative transcriptional regulation of aromatase expression in human breast cancer tissue". J. Steroid Biochem. Mol. Biol. 95 (1–5): 17–23. doi:10.1016/j.jsbmb.2005.04.002. PMID 15955695.
- Lambard S, Silandre D, Delalande C; et al. (2005). "Aromatase in testis: expression and role in male reproduction". J. Steroid Biochem. Mol. Biol. 95 (1–5): 63–9. doi:10.1016/j.jsbmb.2005.04.020. PMID 16019206.
- Bulun SE, Imir G, Utsunomiya H; et al. (2005). "Aromatase in endometriosis and uterine leiomyomata". J. Steroid Biochem. Mol. Biol. 95 (1–5): 57–62. doi:10.1016/j.jsbmb.2005.04.012. PMID 16024248.
- Lambard S, Carreau S (2005). "Aromatase and oestrogens in human male germ cells". Int. J. Androl. 28 (5): 254–9. doi:10.1111/j.1365-2605.2005.00546.x. PMID 16128984.
- Ellem SJ, Risbridger GP (2006). "Aromatase and prostate cancer". Minerva Endocrinol. 31 (1): 1–12. PMID 16498360.
- Brueggemeier RW, Díaz-Cruz ES (2006). "Relationship between aromatase and cyclooxygenases in breast cancer: potential for new therapeutic approaches". Minerva Endocrinol. 31 (1): 13–26. PMID 16498361.
- Jongen VH, Hollema H, Van Der Zee AG, Heineman MJ (2006). "Aromatase in the context of breast and endometrial cancer. A review". Minerva Endocrinol. 31 (1): 47–60. PMID 16498363.
- Hiltunen M, Iivonen S, Soininen H (2006). "Aromatase enzyme and Alzheimer's disease". Minerva Endocrinol. 31 (1): 61–73. PMID 16498364.
External links
- "U.S. study of gay sheep may shed light on sexuality", via WikiNews, 15 August 2005.