Achromatopsia: Difference between revisions
| Line 766: | Line 766: | ||
==Cultural references== | ==Cultural references== | ||
In approximately 1775 Typhoon Lengkieki struck and devastated the Micronesian atoll of Pingelap. The | In approximately 1775 Typhoon Lengkieki struck and devastated the Micronesian atoll of Pingelap. The typhoon and ensuing [[famine]] left only around 20 survivors, one of whom was heterozygous for achromatopsia. Four generations after this [[population bottleneck]] the prevalence of achromatopsia is 5% with a further 30% as carriers. The people of this region have termed achromatopsia "maskun", which literally means "not see" in Pingelapese. This unusual population drew [[neurologist]] [[Oliver Sacks]] to the island for which he wrote his 1997 book, ''The island of the colour-blind''.<ref name="Hussels_1972"/><ref name="Sack_1997">{{cite book|title=The Island of the Colour-blind|first=Oliver|last=Sacks|authorlink=Oliver Sacks|year=1997|publisher=[[Picador (imprint)|Picador]]|isbn=0-330-35887-1}}</ref> | ||
==References== | ==References== | ||
Revision as of 19:00, 2 January 2009
| Achromatopsia | |
| ICD-10 | H53.5 |
|---|---|
| ICD-9 | 368.54 |
| OMIM | 216900 262300 139340 |
| DiseasesDB | 83 |
| MeSH | D003117 |
|
WikiDoc Resources for Achromatopsia |
|
Articles |
|---|
|
Most recent articles on Achromatopsia Most cited articles on Achromatopsia |
|
Media |
|
Powerpoint slides on Achromatopsia |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Achromatopsia at Clinical Trials.gov Trial results on Achromatopsia Clinical Trials on Achromatopsia at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Achromatopsia NICE Guidance on Achromatopsia
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Achromatopsia Discussion groups on Achromatopsia Patient Handouts on Achromatopsia Directions to Hospitals Treating Achromatopsia Risk calculators and risk factors for Achromatopsia
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Achromatopsia |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Achromatopsia (ACHM), is a medical syndrome that exhibits symptoms relating to at least five separate individual diseases. Although the term may refer to acquired disorders such as color agnosia and cerebral achromatopsia, it typically refers to an autosomal recessive congenital color vision disorder, the inability to perceive color AND to achieve satisfactory visual acuity at high light levels (typically exterior daylight). The syndrome is also present in an incomplete form which is more properly defined as dyschromatopsia. The only estimate of its relative occurrence of 1:30,000 in the general population dates from the 1960s or earlier.
There is some discussion as to whether achromats can see color or not. As illustrated in "The Island of the Colorblind" by Oliver Sacks, some achromats cannot see color. With 5 different genes currently known to cause similar symptoms, it may be that some do see marginal levels of color differentiation due to different gene characteristics. With such small sample sizes and low response rates, it is difficult to accurately diagnose the 'typical achromatic conditions'. If the light level during testing is optimized for them, they may achieve corrected visual acuity of 20/100 to 20/150 at lower light levels. One common trait is hemeralopia or blindness in full sun.
The five symptoms associated with achromatopsia/dyschromatopsia are:
- Achromatopia
- Amblyopia (reduced visual acuity)
- Hemeralopia (with the subject exhibiting photophobia)
- Nystagmus
- Iris operating abnormalities
A sixth symptom associated with achromatopsia/dychromatopsia is seldom reported. Many sufferers are unaware of the three-dimensional aspect of their visual system. They frequently fail to observe any of the stereographic features of a scene.
The syndrome of achromatopsia/dyschromatopsia is poorly described in current medical and neuro-ophthalmological texts. It became a common term following the popular book by the neuroscientist Oliver Sacks, "The Island of the Colorblind," in 1997. Up to that time most color-blind subjects were described as achromats or achromatopes. Those with a lesser degree of color perception abnormality were described as either protanopes, deuteranopes or tetartanopes (historically tritanopes).
Achromatopsia has also been called rod monochromacy and total congenital color blindness. Individuals with the congenital form of this disorder show complete absence of cone cell activity via electroretinography at high light levels. There are at least four genetic causes of congenital ACHM, two of which involve cyclic nucleotide-gated ion channels (ACHM2/ACHM3), a third involves the cone photoreceptor transducin (GNAT2, ACHM4), and the last remains unknown.
Classification
- Acquired achromatopsia (Cerebral achromatopsia)
- Congenital/inherited achromatopsia
- Complete/typical achromatopsia
- Incomplete/atypical achromatopsia/dyschromatopsia
- Other terms not elsewhere defined in Wikipedia
- Achromatopia– The complete lack of the perception of color in a subject.
- Amblyopia–Defined conceptually by Duke-Elder (1973) as a monocular acuity deficit which is not due to refractive error or any organic abnormality. A neural condition. Poor spatial performance of the precision optical servomechanism of the eyes at nominal illumination levels without any morphological cause. Lazy eye.
- Hemeralopia–Reduced visual capacity in bright light. Colloquially, day-blindness.
- Nystagmus–This term is used variously to describe both normal and pathological conditions related to the oculomotor system. In the current context, it is a pathological condition involving an uncontrolled oscillatory movement of the eyes during which the amplitude of oscillation is quite noticeable and the frequency of the oscillation tends to be quite low.
- Photophobia–The avoidance of bright light by those suffering from hemeralopia.
Signs and symptoms
The syndrome is frequently noticed first in children around six months of age by their photophobic activity and/or their nystagmus. The nystagmus becomes less noticeable with age but the other symptoms of the syndrome become more relevant as school age approaches. Visual acuity and stability of the eye motions generally improve during the first 6-7 years of life (but remain near 20/200). The congenital forms of the disease are considered stationary and do not worsen with age.
Complete Achromatopsia
Aside from a complete inability to discriminate colors, individuals with complete achromatopsia have a number of other ophthalmologic aberrations. Included among these aberrations are greatly decreased visual acuity (<0.1 or 20/200) in daylight Hemeralopia, nystagmus, and severe photophobia. The fundus of the eye appears completely normal.
Incomplete Achromatopsia (Dyschromatopsia)
In general, symptoms of incomplete achromatopsia are similar to those of complete achromatopsia except in a diminished form. Individuals with incomplete achromatopsia have reduced visual acuity with or without nystagmus or photophobia. Furthermore, these individuals show only partial impairment of cone cell function but again have retained rod cell function.
Cause
Acquired Achromatopsia
Acquired achromatopsia/dyschromatopsia is a condition associated with damage to the diencephalon (primarily the thalamus of the mid brain) or the cerebral cortex (the new brain).
Thalamic achromatopsia/dyschromatopsia is caused by damage to the thalamus, a portion of the brain stem. It is most frequently caused by tumor growth since the thalamus is well protected from external damage.
Cerebral achromatopsia is a form of acquired color blindness that is caused by damage to the cerebral cortex of the brain, rather than abnormalities in the cells of the eye's retina. It is most frequently caused by physical trauma, hemorrhage or tumor tissue growth.
Congenital Achromatopsia
The known causes of the congenital forms of achromatopsia are all due to malfunction of the retinal phototransduction pathway. Specifically, this form of ACHM seems to result from the inability of cone cells to properly respond to light input by hyperpolarizing. Known genetic causes of this are mutations in the cone cell cyclic nucleotide-gated ion channels CNGA3 (ACHM2) and CNGB3 (ACHM3) as well as the cone cell transducin, GNAT2 (ACHM4).
Pathophysiology
The hemeralopic aspect of ACHM can be diagnosed non-invasively using electroretinography. The response at low (scotopic) and median (mesotopic) light levels will be normal but the response under high light level (photopic) conditions will be absent. The mesotopic level is approximately 100 times lower than the clinical level used for the typical high level electroretinogram. When as described, the condition is due to a saturation in the neural portion of the retina and not due to the absence of the photoreceptors per se.
Pathophysiology based on an electrolytic theory of the neural system
The electrolytic theory of the neural system provides a more functional description of achromatopsia/dyschromatopsia.[1] It describes ten specific locations for the individual diseases contributing to the diagnosis of achromatopsia. These locations are described in slides 9 through 15 of the Briefing to the 2008 Achromatopsia Conference. This Briefing is available in PDF format[2].
A summary slide is shown below. It is a block diagram of the visual subsystem showing potential failure locations leading to Achromatopsia/Dyschromatopsia. These locations include six within the nominal retina, five within the diencephalon of the midbrain and at least three in the cerebral cortex. The fifth location within the diencephalon is not shown (interrupting the R-channel) because it leads to a more serious condition not discussed here.
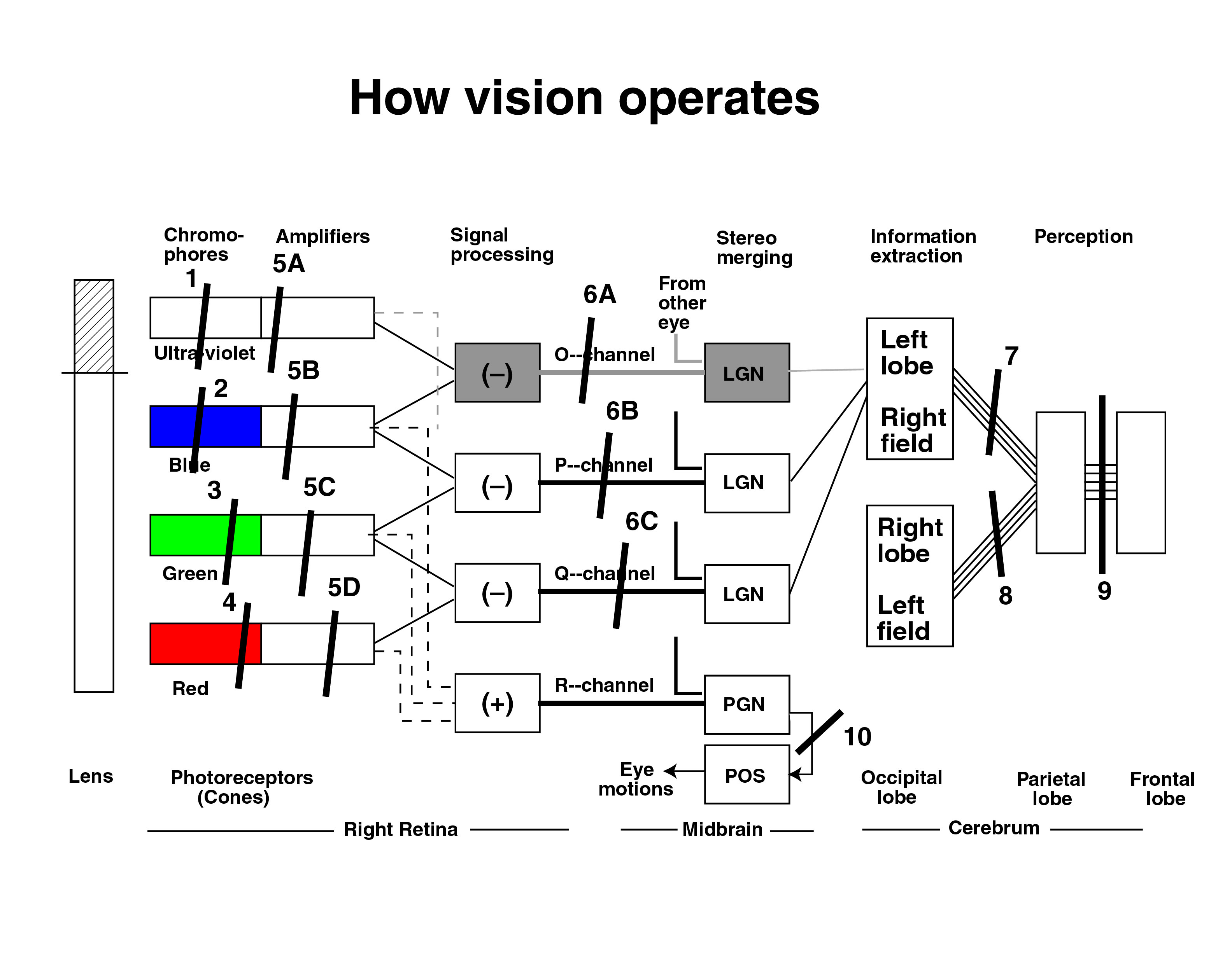
- Failure, Result
- 2 or 5B, Tritanopia (rare)
- 3 or 5C, Pentanopia (rarer)
- 4 or 5D, Protanopia
- 6B, Blue/Green dyschrom. (tetartanopia)
- 6C, Red/Green dyschrom. (deuteranopia)
- 7, Right hemiachromatopia
- 8, Left hemiachromatopia
- 9, Cerebral Achromatopia
- 10, Nystagmus, Strabismus a/o Amblyopia
- Based on the well documented spectral sensitivity of Nordby, himself a vision researcher, failure of the actual photoreceptors of the retina (#2,3 or 4) are an infrequent cause of achromatopsia and/or achromatopia.[3] A failure at #4 is more common in men and results in the (partial) colorblind condition called protanopia.
- While little known, the ultraviolet channel shown is critically important in the sensing of the violet shades of the visual spectrum between 400 nanometers and 437 nanometers. It is well documented in the literature. See page 135 of the earlier Fulton reference. The lens of the eye truncates the spectrum near 400 nanometers as shown. A failure at #1, 5A or 6A would likely go unnoticed outside the laboratory and probably has not been reported in the literature.
Pathophysiology based on a chemical theory of the neural system
In general, the molecular pathomechanism of ACHM is either the inability to properly control or respond to altered levels of cGMP. cGMP is particularly important in visual perception as its level controls the opening of cyclic nucleotide-gated ion channels (CNGs). Decreasing the concentration of cGMP results in closure of CNGs and resulting hyperpolarization and cessation of glutamate release. Native retinal CNGs are comprised of 2 α- and 2 β-subunits, which are CNGA3 and CNGB3, respectively, in cone cells. When expressed alone, CNGB3 cannot produce functional channels, whereas this is not the case for CNGA3. Coassembly of CNGA3 and CNGB3 produces channels with altered membrane expression, ion permeability (Na+ vs. K+ and Ca2+), relative efficacy of cAMP/cGMP activation, decreased outward rectification, current flickering, and sensitivity to block by L-cis-diltiazem. Mutations tend to result in the loss of CNGB3 function or gain of function (often increased affinity for cGMP) of CNGA3. cGMP levels are controlled by the activity of the cone cell transducin, GNAT2. Mutations in GNAT2 tend to result in a truncated and, presumably, non-functional protein, thereby preventing alteration of cGMP levels by photons. There is a positive correlation between the severity of mutations in these proteins and the completeness of the achromatopsia phenotype.
ACHM2
While some mutations in CNGA3 result in truncated and, presumably, non-functional channels this is largely not the case. While few mutations have received in-depth study, see table 1, at least one mutation does result in functional channels. Curiously, this mutation, T369S, produces profound alterations when expressed without CNGB3. One such alteration is decreased affinity for Cyclic guanosine monophosphate. Others include the introduction of a sub-conductance, altered single-channel gating kinetics, and increased calcium permeability. When mutant T369S channels coassemble with CNGB3, however, the only remaining aberration is increased calcium permeability.[4] While it is not immediately clear how this increase in Ca2+ leads to ACHM, one hypothesis is that this increased current decreases the signal-to-noise ratio. Other characterized mutations, such as Y181C and the other S1 region mutations, result in decreased current density due to an inability of the channel to traffic to the surface.[5] Such loss of function will undoubtedly negate the cone cell's ability to respond to visual input and produce achromatopsia. At least one other missense mutation outside of the S1 region, T224R, also leads to loss of function.[4]
| Mutation | Region | Functional? (known or predicted) |
Effect | References | |
|---|---|---|---|---|---|
| Nucleotide | Amino acid | ||||
| c.C67T | p.R23X | N-Term | No? | [6] | |
| c.148insG | p.I50DfsX59 | N-Term | No? | [7] | |
| c.A485T | p.D162V | N-Term | [7] | ||
| c.C488T | p.P163L | N-Term | [7], [8] | ||
| c.A542G | p.Y181C | S1 | No | Does not properly traffic out of the endoplasmic reticulum | [5],[7] |
| c.A544T | p.N182Y | S1 | No | Does not properly traffic out of the endoplasmic reticulum | [5],[7] |
| c.C556T | p.L186F | S1 | No | Does not properly traffic out of the endoplasmic reticulum | [5],[7] |
| c.G572A | p.C191Y | S1-2 | No | Does not properly traffic out of the endoplasmic reticulum | [5],[7] |
| c.G580A | p.E194K | S1-2 | No | Does not properly traffic out of the endoplasmic reticulum | [7] |
| c.C586T | p.G196X | S1-2 | No? | [6] | |
| c.C661T | p.R221X | S2 | No? | [6] | |
| c.C667T | p.R223W | S2-3 | [6],[7] | ||
| c.C671G | p.T224R | S2-3 | No | No current | [4],[7] |
| c.G778A | p.D260N | S3 | [7] | ||
| c.G800A | p.G267D | S3-4 | [7] | ||
| c.C829T | p.R277C | S4 | [7] | ||
| c.G830A | p.R277H | S4 | [7] | ||
| c.C847T | p.R283Q | S4 | [7], [8] | ||
| c.G848A | p.R283W | S4 | [7], [8] | ||
| c.C872G | p.T291R | S4 | [7], [8] | ||
| c.934_936del | p.312delI | S5 | [7] | ||
| c.G947A | p.W316X | S5 | No? | [7] | |
| c.T1021C | p.S341P | Pore | [7] | ||
| c.C1106G | p.T369S | pore | Yes | Increased calcium influx | [4], [7] |
| c.C1114T | p.P372S | Pore | [7] | ||
| c.T1139C | p.F380S | Pore | [7] | ||
| c.T1217C | p.M406T | S6 | [7] | ||
| c.C1228T | p.R410W | C-term | [7], [8] | ||
| c.C1279T | p.R427C | C-term | [7] | ||
| c.C1306T | p.R436W | C-term | [6], [7] | ||
| c.G1320A | p.W440X | C-term | No? | [7] | |
| c.1350insG | p.V451GfsX453 | C-term | No? | [7] | |
| c.A1412G | p.N471S | C-term | [7] | ||
| c.1443insC | p.I482HfsX5 | C-term | No? | [6] | |
| c.A1454T | p.D485V | C-term | [7] | ||
| c.G1529C | p.C510S | cNMP | [7] | ||
| c.G1538A | p.G513E | cNMP | [7] | ||
| c.G1547A | p.G516E | cNMP | [7] | ||
| c.T1565C | p.I522T | cNMP | [7] | ||
| c.G1574A | p.G525D | cNMP | [7] | ||
| c.G1585A | p.V529M | cNMP | [7], [8] | ||
| c.C1609T | p.Q537X | cNMP | No? | [6], [7] | |
| c.C1641A | p.F547L | cNMP | [7], [8] | ||
| c.G1642A | p.G548R | cNMP | [6] | ||
| c.G1669A | p.G557R | cNMP | [7], [8] | ||
| c.G1688A | p.R563H | cNMP | [7] | ||
| c.C1694T | p.T565M | cNMP | [7] | ||
| c.G1706A | p.R569H | cNMP | [6], [7] | ||
| c.A1718G | p.Y573C | cNMP | [7] | ||
| c.G1777A | p.E593K | cNMP | [7] | ||
| c.C1963T | p.Q655X | C-term | [7] | ||
| Abbreviations: SX, transmembrane segment number X; SX-Y, linker region between transmembrane segments X and Y; cNMP, cyclic nucleotide (cAMP or cGMP) binding region. | |||||
ACHM3
While very few mutations in CNGB3 have been characterized, the vast majority of them result in truncated channels that are presumably non-functional, table 2. This will largely result in haploinsufficiency, though in some cases the truncated proteins may be able to coassemble with wild-type channels in a dominant negative fashion. The most prevalent ACHM3 mutation, T383IfsX12, results in a non-functional truncated protein that does not properly traffic to the cell membrane.[9][10] The three missense mutations that have received further study show a number of aberrant properties, with one underlying theme. The R403Q mutation, which lies in the pore region of the channel, results in an increase in outward current rectification, versus the largely linear current-voltage relationship of wild-type channels, concomitant with an increase in cGMP affinity.[10] The other mutations show either increased (S435F) or decreased (F525N) surface expression but also with increased affinity for cAMP and cGMP.[9][10] It is the increased affinity for cGMP and cAMP in these mutants that is likely the disease-causing change. Such increased affinity will result in channels that are insensitive to the slight concentration changes of cGMP due to light input into the retina.
| Mutation | Region | Functional? (known or predicted) |
Effect | References | |
|---|---|---|---|---|---|
| Nucleotide | Amino acid | ||||
| c.29_30insA | p.K10fsX9 | N-Term | No? | [11] | |
| c.C112T | p.Q38X | N-Term | No? | [11] | |
| c.C391T | p.Q131X | N-Term | No? | [11] | |
| c.A442G | p.K148E | N-Term | [12] | ||
| c.446_447insT | p.K149NfsX29 | N-Term | No? | [12] | |
| c.C467T | p.S156F | N-Term | [11] | ||
| c.595delG | p.E199SfsX2 | N-Term | No? | [6] | |
| c.C607T | p.R203X | N-Term | No? | [11], [13] | |
| c.G644-1C | Splicing | No? | [11] | ||
| c.C646T | p.R216X | N-Term | No? | [11] | |
| c.682_683insG | p.A228GfsX2 | S1 | No? | [11] | |
| c.G702A | p.W234X | S1 | No? | [11] | |
| c.706_707delinsTT | p.I236FfsX25 | S1 | No? | [11] | |
| c.819_826del | p.P273fsX13 | S2-3 | No? | [13], [14] | |
| c.882_892delinsT | p.R295QfsX9 | S2-3 | No? | [11] | |
| c.C926T | p.P309L | S3 | [11] | ||
| c.T991-3G | Splicing | No? | [11] | ||
| c.G1006T | p.E336X | S4 | No? | [11], [13] | |
| c.C1063T | p.R355X | S5 | No? | [11] | |
| c.G1119A | p.W373X | S5 | No? | [11] | |
| c.1148delC | p.T383IfsX12 | Pore | No | Does not traffic to the surface | [9], [10], [11], [6], [13], [14] |
| c.G1208A | p.R403Q | Pore | Yes | Increased outward rectification, increased cGMP affinity | [10] |
| c.G1255T | p.E419X | Pore | No? | [11] | |
| c.1298_1299del | p.V433fsX27 | S6 | No? | [11] | |
| c.C1304T | p.S435F | S6 | Yes | Increased affinity for cAMP and cGMP, decreased surface expression, altered ion permeability, decreased single channel conductance, decreased sensitivity for diltiazem | [9], [11], [13], [14] |
| c.C1432T | p.R478X | C-term | No? | [11] | |
| c.G1460A | p.W487X | C-term | No? | [11] | |
| c.1573_1574delinsTT | p.F525N | C-term | Yes | Increased surface expression in oocytes, decreased outward rectification, increased cGMP and cAMP affinity | [10], [6] |
| c.G1578+1A | Splicing | No? | [11], [13] | ||
| c.T1635A | p.Y545X | cNMP | No? | [11] | |
| c.G1781+1C | Splicing | No? | [11] | ||
| c.G1781+1A | Splicing | No? | [11] | ||
| c.2160_2180del | p.720_726del | C-term | [11] | ||
| Abbreviations: SX, transmembrane segment number X; SX-Y, linker region between transmembrane segments X and Y; cNMP, cyclic nucleotide (cAMP or cGMP) binding region. | |||||
ACHM4
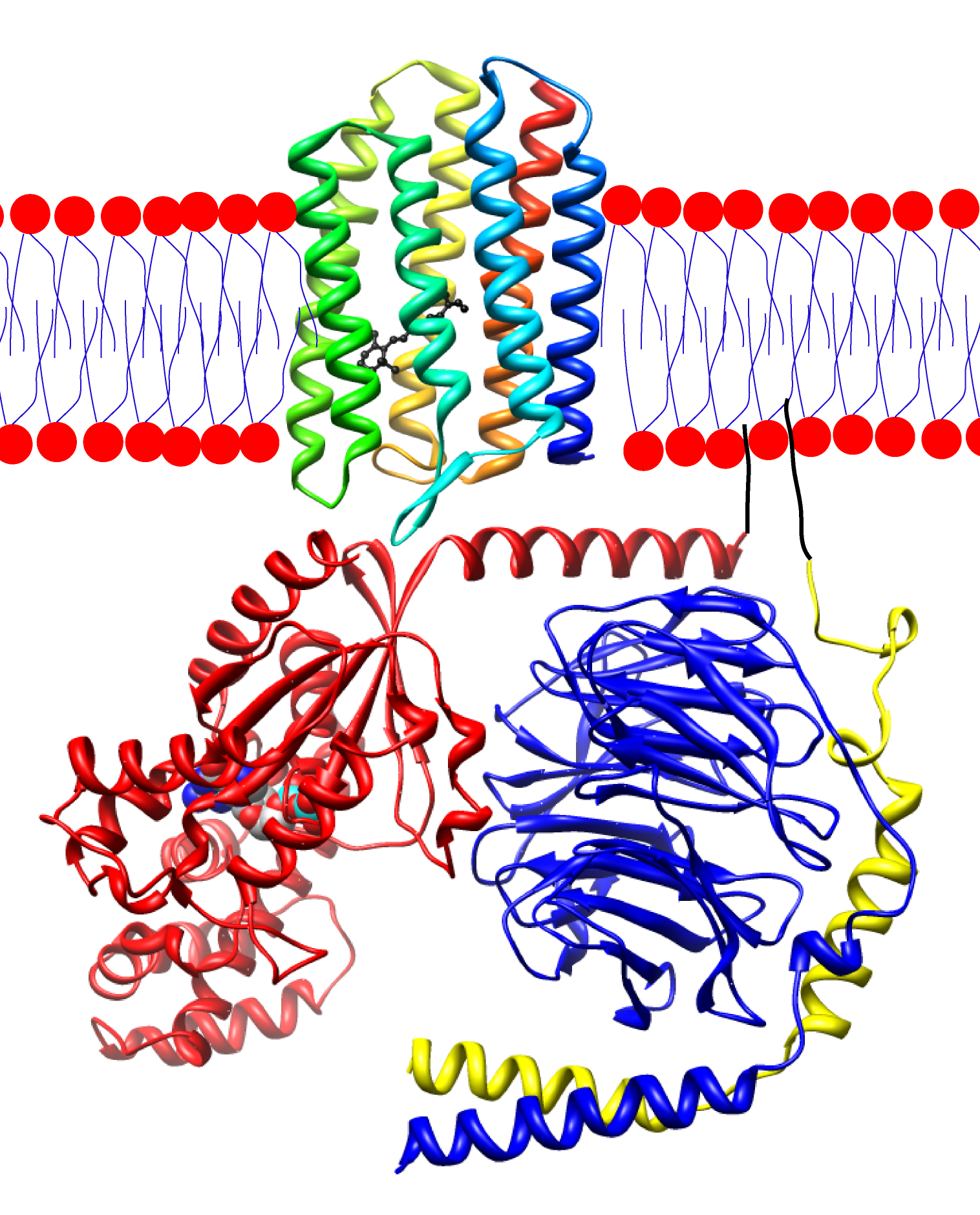
Upon activation of by light, rhodopsin causes the exchange of GDP for GTP in the guanine nucleotide binding protein (G-protein) α-transducing activity polypeptide 2 (GNAT2). This causes the release of the activated α-subunit from the inhibitory β/γ-subunits. This α-subunit then activates a phosphodiesterase that catalyzes the conversion of cGMP to GMP, thereby reducing current through CNG3 channels. As this process is absolutely vital for proper color processing it is not surprising that mutations in GNAT2 lead to achromatopsia. The known mutations in this gene, table 3, all result in truncated proteins. Presumably, then, these proteins are non-functional and, consequently, rhodopsin that has been activated by light does not lead to altered cGMP levels or photoreceptor membrane hyperpolarization.
| Mutation | Functional? (predicted) |
References | |
|---|---|---|---|
| Nucleotide | Amino acid | ||
| c.C235T | p.Q79X | No? | [15] |
| c.285_291del | p.Y95fsX61 | No? | [15] |
| IVS3+365_IVS4+974del | p.A101fsX12 | No? | [15] |
| c.503_504insT | p.L168fsX3 | No? | [15] |
| c.802_803insTCAA | p.L268fsX9 | No? | [15] |
| c.955del | p.I319SfsX5 | No? | [15] |
Epidemiology
Achromatopsia is a relatively uncommon disorder, with a prevalence of 1 in 30,000 people (0.0033%).[16] However, in the small Micronesian atoll of Pingelap approximately 5% of the atoll's 3000 inhabitants are afflicted.[17][18]
Cultural references
In approximately 1775 Typhoon Lengkieki struck and devastated the Micronesian atoll of Pingelap. The typhoon and ensuing famine left only around 20 survivors, one of whom was heterozygous for achromatopsia. Four generations after this population bottleneck the prevalence of achromatopsia is 5% with a further 30% as carriers. The people of this region have termed achromatopsia "maskun", which literally means "not see" in Pingelapese. This unusual population drew neurologist Oliver Sacks to the island for which he wrote his 1997 book, The island of the colour-blind.[18][19]
References
- ↑ Fulton, James (2004). Biological Vision: A 21st Century Tutorial. Trafford. ISBN 141201917-6.
- ↑ Briefing from 2008 Achromatopsia Conference by James Fulton
- ↑ Nordby K, Stabell B, Stabell U (1984). "Dark-Adaptation of the Human Rod System". Vision Research. 24 (8): 841–849.
- ↑ 4.0 4.1 4.2 4.3 Tränkner D, Jägle H, Kohl S; et al. (2004). "Molecular basis of an inherited form of incomplete achromatopsia". J. Neurosci. 24 (1): 138–47. doi:10.1523/JNEUROSCI.3883-03.2004. PMID 14715947.
- ↑ 5.0 5.1 5.2 5.3 5.4 Patel KA, Bartoli KM, Fandino RA; et al. (2005). "Transmembrane S1 mutations in CNGA3 from achromatopsia 2 patients cause loss of function and impaired cellular trafficking of the cone CNG channel". Invest. Ophthalmol. Vis. Sci. 46 (7): 2282–90. doi:10.1167/iovs.05-0179. PMID 15980212.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 Johnson S, Michaelides M, Aligianis IA; et al. (2004). "Achromatopsia caused by novel mutations in both CNGA3 and CNGB3". J. Med. Genet. 41 (2): e20. doi:10.1136/jmg.2003.011437. PMID 14757870.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 7.20 7.21 7.22 7.23 7.24 7.25 7.26 7.27 7.28 7.29 7.30 7.31 7.32 7.33 7.34 7.35 7.36 7.37 7.38 7.39 7.40 7.41 7.42 7.43 7.44 7.45 Wissinger B, Gamer D, Jägle H; et al. (2001). "CNGA3 mutations in hereditary cone photoreceptor disorders". Am. J. Hum. Genet. 69 (4): 722–37. doi:10.1086/323613. PMID 11536077.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Kohl S, Marx T, Giddings I; et al. (1998). "Total colourblindness is caused by mutations in the gene encoding the alpha-subunit of the cone photoreceptor cGMP-gated cation channel". Nat. Genet. 19 (3): 257–9. doi:10.1038/935. PMID 9662398.
- ↑ 9.0 9.1 9.2 9.3 Peng C, Rich ED, Varnum MD (2003). "Achromatopsia-associated mutation in the human cone photoreceptor cyclic nucleotide-gated channel CNGB3 subunit alters the ligand sensitivity and pore properties of heteromeric channels". J. Biol. Chem. 278 (36): 34533–40. doi:10.1074/jbc.M305102200. PMID 12815043.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Bright SR, Brown TE, Varnum MD (2005). "Disease-associated mutations in CNGB3 produce gain of function alterations in cone cyclic nucleotide-gated channels". Mol. Vis. 11: 1141–50. PMID 16379026.
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 11.15 11.16 11.17 11.18 11.19 11.20 11.21 11.22 11.23 11.24 11.25 11.26 Kohl S, Varsanyi B, Antunes GA; et al. (2005). "CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia". Eur. J. Hum. Genet. 13 (3): 302–8. doi:10.1038/sj.ejhg.5201269. PMID 15657609.
- ↑ 12.0 12.1 Rojas CV, María LS, Santos JL, Cortés F, Alliende MA (2002). "A frameshift insertion in the cone cyclic nucleotide gated cation channel causes complete achromatopsia in a consanguineous family from a rural isolate". Eur. J. Hum. Genet. 10 (10): 638–42. doi:10.1038/sj.ejhg.5200856. PMID 12357335.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Kohl S, Baumann B, Broghammer M; et al. (2000). "Mutations in the CNGB3 gene encoding the beta-subunit of the cone photoreceptor cGMP-gated channel are responsible for achromatopsia (ACHM3) linked to chromosome 8q21". Hum. Mol. Genet. 9 (14): 2107–16. doi:10.1093/hmg/9.14.2107. PMID 10958649.
- ↑ 14.0 14.1 14.2 Sundin OH, Yang JM, Li Y; et al. (2000). "Genetic basis of total colourblindness among the Pingelapese islanders". Nat. Genet. 25 (3): 289–93. doi:10.1038/77162. PMID 10888875.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Kohl S, Baumann B, Rosenberg T; et al. (2002). "Mutations in the cone photoreceptor G-protein alpha-subunit gene GNAT2 in patients with achromatopsia". Am. J. Hum. Genet. 71 (2): 422–5. doi:10.1086/341835. PMID 12077706.
- ↑ Francois, J (1961). Heredity in ophthalmology. St. Louis: Mosby.
- ↑ Brody JA, Hussels I, Brink E, Torres J (1970). "Hereditary blindness among Pingelapese people of Eastern Caroline Islands". Lancet. 1 (7659): 1253–7. PMID 4192495.
- ↑ 18.0 18.1 Hussels IE, Morton NE (1972). "Pingelap and Mokil Atolls: achromatopsia". Am. J. Hum. Genet. 24 (3): 304–9. PMID 4555088.
- ↑ Sacks, Oliver (1997). The Island of the Colour-blind. Picador. ISBN 0-330-35887-1.
See also
External links
- The Achromatopsia Network
- The Achromatopsia Group
- Achromatopsia and the underlying bioelectrochemistry
- Fulton, James (2005) Processes in Biological Vision
ca:Acromatòpsia et:Akromatopsia gl:Acromatopsia it:Acromatopsia nl:Achromatopsie