Esmolol adverse reactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The following adverse reaction rates are based on use of BREVIBLOC (Esmolol Hydrochloride) in clinical trials involving 369 patients with supraventricular tachycardia and over 600 intraoperative and postoperative patients enrolled in clinical trials. Most adverse effects observed in controlled clinical trial settings have been mild and transient. The most important and common adverse effect has been hypotension [see Warnings and Precautions (5.1)]. Deaths have been reported in post-marketing experience occurring during complex clinical states where BREVIBLOC was presumably being used simply to control ventricular rate [see Warnings and Precautions (5.5)].
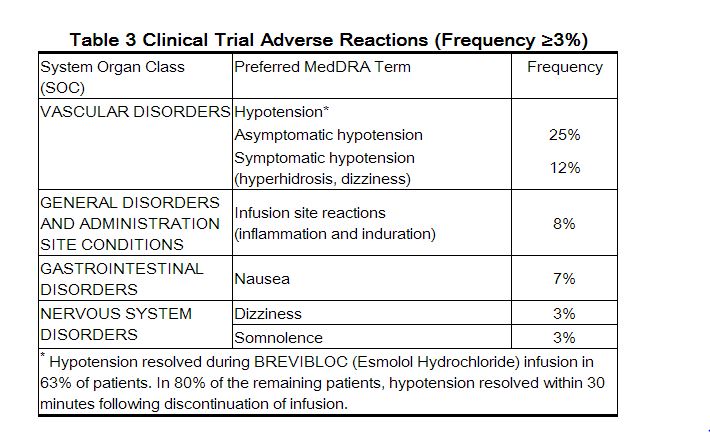 |
Clinical Trial Adverse Reactions (Frequency <3%)
Psychiatric Disorders
Confusional state and agitation (~2%) Anxiety, depression and abnormal thinking (<1%)
Nervous System Disorders
Headache (~ 2%) Paresthesia, syncope, speech disorder, and lightheadedness (<1%) Convulsions (<1%), with one death
Vascular Disorders
Peripheral ischemia (~1%) Pallor and flushing (<1%)
Gastrointestinal Disorders
Vomiting (~1%) Dyspepsia, constipation, dry mouth, and abdominal discomfort (<1%)
Renal and Urinary Disorders
Urinary retention (<1%)
Post-Marketing Experience
In addition to the adverse reactions reported in clinical trials, the following adverse reactions have been reported in the post-marketing experience. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or to establish a causal relationship to drug exposure.
Cardiac Disorders
Cardiac arrest, Coronary arteriospasm
Skin and Subcutaneous Tissue Disorders
Angioedema, Urticaria, Psoriasis[1]
References
Adapted from the FDA Package Insert.