Esmolol
{{DrugProjectFormSinglePage |authorTag=Alonso Alvarado, M.D. [1] |genericName=Esmolol |aOrAn=a |drugClass=beta-adrenergic blocker |indication=supraventricular tachycardiaor noncompensatory sinus tachycardia, intraoperative and postoperative tachycardia and/or hypertension |adverseReactions=hypotension, injection site pain, nausea |blackBoxWarningTitle=Warning Title |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult======Supraventricular Tachycardia or Noncompensatory Sinus Tachycardia=====
- Dosing Information
- Esmolol is administered by continuous intravenous infusion with or without a loading dose. Additional loading doses and/or titration of the maintenance infusion (step-wise dosing) may be necessary based on desired ventricular response.
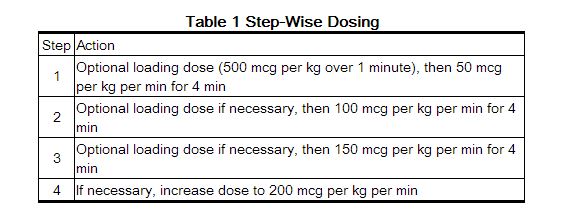
- In the absence of loading doses, continuous infusion of a single concentration of esmolol reaches pharmacokinetic and pharmacodynamic steady-state in about 30 minutes.
- The effective maintenance dose for continuous and step-wise dosing is 50 to 200 mcg per kg per minute, although doses as low as 25 mcg per kg per minute have been adequate. Dosages greater than 200 mcg per kg per minute provide little added heart rate lowering effect, and the rate of adverse reactions increases.
- Maintenance infusions may be continued for up to 48 hours.
Intraoperative and Postoperative Tachycardia and Hypertension
- Dosing Information
- In this setting it is not always advisable to slowly titrate to a therapeutic effect. Therefore two dosing options are presented: immediate control and gradual control.
Immediate Control
- Administer 1 mg per kg as a bolus dose over 30 seconds followed by an infusion of 150 mcg per kg per min if necessary.
- Adjust the infusion rate as required to maintain desired heart rate and blood pressure. Refer to Maximum Recommended Doses below.
Gradual Control
- Administer 500 mcg per kg as a bolus dose over 1 minute followed by a maintenance infusion of 50 mcg per kg per min for 4 minutes.
- Depending on the response obtained, continue dosing as outlined for supraventricular tachycardia. Refer to Maximum Recommended Doses below.
Maximum Recommended Doses
- For the treatment of tachycardia, maintenance infusion dosages greater than 200 mcg per kg per min are not recommended; dosages greater than 200 mcg per kg per min provide little additional heart rate-lowering effect, and the rate of adverse reactions increases.
- For the treatment of hypertension, higher maintenance infusion dosages (250-300 mcg per kg per min) may be required. The safety of doses above 300 mcg per kg per minute has not been studied.
Transition from esmolol Injection Therapy to Alternative Drugs
- After patients achieve adequate control of the heart rate and a stable clinical status, transition to alternative antiarrhythmic drugs may be accomplished.
- When transitioning from esmolol to alternative drugs, the physician should carefully consider the labeling instructions of the alternative drug selected and reduce the dosage of esmolol as follows:
- Thirty minutes following the first dose of the alternative drug, reduce the esmolol infusion rate by one-half (50%).
- After administration of the second dose of the alternative drug, monitor the patient’s response and if satisfactory control is maintained for the first hour, discontinue the esmolol infusion.
Directions for Use
- Esmolol injection is available in a pre-mixed bag and ready-to-use vial. Esmolol is not compatible with Sodium Bicarbonate (5%) solution (limited stability) or furosemide (precipitation).
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
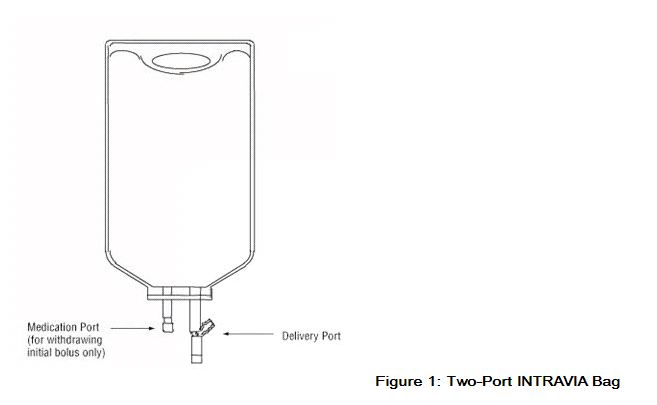
Premixed Bag
- The medication port is to be used solely for withdrawing an initial bolus from the bag.
- Use aseptic technique when withdrawing the bolus dose.
- Do not add any additional medications to the bag.
Ready-to-Use Vial
- The Ready-to-use Vial may be used to administer a loading dosage by hand-held syringe while the maintenance infusion is being prepared.
|offLabelAdultGuideSupport======Atrial arrhythmia, Adjunct=====
- Developed by: AHA/ACC/HRS
- IV bolus of 500 mcg/kg administered over 1 min followed by IV infusion of 50–300 mcg/kg/min[1]
|offLabelAdultNoGuideSupport======Acute Myocardial Infarction=====
- Dosing Information
- Initial dose of 500 mcg/kg/min over 1 minute, then a titrated infusion to a dose of 50 to 300 mcg/kg/min administered over 4 minutes.[2]
Anesthesia in Surgical procedure
- Dosing Information
- 1 mg/kg bolus followed by 250 mcg/kg/min in association with propofol.[3]
Aortic surgery, aortic dissection
Electroconvulsive therapy; Adjunct
- Dosing Information
- 80 mg IV[4]
Myocardial protection in Heart Surgery
- Dosing Information
- 100 mg bolus into the aortic root administered followed by a 10 to 15 mg/min infusion as many times as required.[5]
Pheochromocytoma
- Dosing Information
- 10 mg/mL.[6]
Thyroid Storm
- Dosing Information
- Initial dose of 500 mcg/kg administered over 1 minute, then 50 mcg/kg/min and titrated up to a maximum of 200 mcg/kg/min in 50 mcg/kg/min increments every 4 minutes.[7]
Unstable Angina
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Esmolol in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Esmolol in pediatric patients. |contraindications=Esmolol is contraindicated in patients with:
- Severe sinus bradycardia: May precipitate or worsen bradycardia resulting in cardiogenic shock and cardiac arrest.
- Heart block greater than first degree: Second- or third-degree atrioventricular block may precipitate or worsen bradycardia resulting in cardiogenic shock and cardiac arrest.
- Sick sinus syndrome: May precipitate or worsen bradycardia resulting in cardiogenic shock and cardiac arrest.
- Decompensated heart failure: May worsen heart failure.
- Cardiogenic shock: May precipitate further cardiovascular collapse and cause cardiac arrest.
- IV administration of cardiodepressant calcium channel blockers (e.g., verapamil) and esmolol in close proximity (i.e., while cardiac effects from the other are still present); fatal cardiac arrests have occurred in patients receiving esmolol and intravenous verapamil.
- Pulmonary hypertension: May precipitate cardiorespiratory compromise.
- Hypersensitivity reactions, including anaphylaxis to esmolol or any of the inactive ingredients of the product (cross-sensitivity between beta blockers is possible).
|warnings======Hypotension=====
Hypotension can occur at any dose but is dose-related. Patients with hemodynamic compromise or on interacting medications are at particular risk. Severe reactions may include loss of consciousness, cardiac arrest, and death. For control of ventricular heart rate, maintenance doses greater than 200 mcg per kg per min are not recommended. Monitor patients closely, especially if pretreatment blood pressure is low. In case of an unacceptable drop in blood pressure, reduce or stop esmolol injection. Decrease of dose or termination of infusion reverses hypotension, usually within 30 minutes.
Bradycardia
Bradycardia, including sinus pause, heart block, severe bradycardia, and cardiac arrest have occurred with the use of esmolol injection. Patients with first-degree atrioventricular block, sinus node dysfunction, or conduction disorders may be at increased risk. Monitor heart rate and rhythm in patients receiving esmolol.
If severe bradycardia develops, reduce or stop esmolol.
Cardiac Failure
Beta blockers, like esmolol injection, can cause depression of myocardial contractility and may precipitate heart failure and cardiogenic shock. At the first sign or symptom of impending cardiac failure, stop esmolol and start supportive therapy.
Intraoperative and Postoperative Tachycardia and/or Hypertension
Monitor vital signs closely and titrate esmolol slowly in the treatment of patients whose blood pressure is primarily driven by vasoconstriction associated with hypothermia.
Reactive Airways Disease
Patients with reactive airways disease should, in general, not receive beta blockers. Because of its relative beta1 selectivity and titratability, titrate esmolol to the lowest possible effective dose. In the event of bronchospasm, stop the infusion immediately; a beta2 stimulating agent may be administered with appropriate monitoring of ventricular rates.
Use in Patients with Diabetes Mellitus and Hypoglycemia
In patients with hypoglycemia, or diabetic patients (especially those with labile diabetes) who are receiving insulin or other hypoglycemic agents, beta blockers may mask tachycardia occurring with hypoglycemia, but other manifestations such as dizziness and sweating may not be masked.
Concomitant use of beta blockers and antidiabetic agents can enhance the effect of antidiabetic agents (blood glucose–lowering).
Infusion Site Reactions
Infusion site reactions have occurred with the use of esmolol injection. They include irritation, inflammation, and severe reactions (thrombophlebitis, necrosis, and blistering), in particular when associated with extravasation. Avoid infusions into small veins or through a butterfly catheter.
If a local infusion site reaction develops, use an alternative infusion site and avoid extravasation.
Use in Patients with Prinzmetal's Angina
Beta blockers may exacerbate anginal attacks in patients with Prinzmetal’s angina because of unopposed alpha receptor–mediated coronary artery vasoconstriction. Do not use nonselective beta blockers.
Use in Patients with Pheochromocytoma
If esmolol is used in the setting of pheochromocytoma, give it in combination with an alpha-blocker, and only after the alpha-blocker has been initiated. Administration of beta-blockers alone in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure from the attenuation of beta-mediated vasodilation in skeletal muscle.
Use in Hypovolemic Patients
In hypovolemic patients, esmolol injection can attenuate reflex tachycardia and increase the risk of hypotension.
Use in Patients with Peripheral Circulatory Disorders
In patients with peripheral circulatory disorders (including Raynaud’s disease or syndrome, and peripheral occlusive vascular disease), esmolol may aggravate peripheral circulatory disorders.
Abrupt Discontinuation of esmolol Injection
Severe exacerbations of angina, myocardial infarction, and ventricular arrhythmias have been reported in patients with coronary artery disease upon abrupt discontinuation of beta blocker therapy. Observe patients for signs of myocardial ischemia when discontinuing esmolol.
Heart rate increases moderately above pretreatment levels 30 minutes after esmolol discontinuation.
Hyperkalemia
Beta blockers, including esmolol, have been associated with increases in serum potassium levels and hyperkalemia. The risk is increased in patients with risk factors such as renal impairment. Intravenous administration of beta blockers has been reported to cause potentially life-threatening hyperkalemia in hemodialysis patients. Monitor serum electrolytes during therapy with esmolol.
Use in Patients with Metabolic Acidosis
Beta blockers, including esmolol, have been reported to cause hyperkalemic renal tubular acidosis. Acidosis in general may be associated with reduced cardiac contractility.
Use in Patients with Hyperthyroidism
Beta-adrenergic blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Abrupt withdrawal of beta blockade might precipitate a thyroid storm; therefore, monitor patients for signs of thyrotoxicosis when withdrawing beta blocking therapy.
Use in Patients at Risk of Severe Acute Hypersensitivity Reactions
When using beta blockers, patients at risk of anaphylactic reactions may be more reactive to allergen exposure (accidental, diagnostic, or therapeutic).
Patients using beta blockers may be unresponsive to the usual doses of epinephrine used to treat anaphylactic or anaphylactoid reactions. |clinicalTrials======Clinical Trials Experience=====
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The following adverse reaction rates are based on use of esmolol in clinical trials involving 369 patients with supraventricular tachycardia and over 600 intraoperative and postoperative patients enrolled in clinical trials. Most adverse effects observed in controlled clinical trial settings have been mild and transient. The most important and common adverse effect has been hypotension. Deaths have been reported in post-marketing experience occurring during complex clinical states where esmolol was presumably being used simply to control ventricular rate
Clinical Trial Adverse Reactions (Frequency <3%)
Psychiatric Disorders
- Confusional state and agitation (~2%)
- Anxiety, depression and abnormal thinking (<1%)
Nervous System Disorders
- Headache (~ 2%)
- Paresthesia, syncope, speech disorder, and lightheadedness (<1%)
- Convulsions (<1%), with one death
Vascular Disorders
- Peripheral ischemia (~1%)
- Pallor and flushing (<1%)
Gastrointestinal Disorders
- Vomiting (~1%)
- Dyspepsia, constipation, dry mouth, and abdominal discomfort (<1%)
Renal and Urinary Disorders
- Urinary retention (<1%)
|postmarketing=In addition to the adverse reactions reported in clinical trials, the following adverse reactions have been reported in the post-marketing experience. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or to establish a causal relationship to drug exposure.
Cardiac Disorders
- Cardiac arrest, Coronary arteriospasm
Skin and Subcutaneous Tissue Disorders
|drugInteractions=Concomitant use of esmolol injection with other drugs that can lower blood pressure, reduce myocardial contractility, or interfere with sinus node function or electrical impulse propagation in the myocardium can exaggerate esmolol's effects on blood pressure, contractility, and impulse propagation. Severe interactions with such drugs can result in, for example, severe hypotension, cardiac failure, severe bradycardia, sinus pause, sinoatrial block, atrioventricular block, and/or cardiac arrest. In addition, with some drugs, beta blockade may precipitate increased withdrawal effects (e.g. clonidine, guanfacine, and monoxide). Esmolol should therefore be used only after careful individual assessment of the risks and expected benefits in patients receiving drugs that can cause these types of pharmacodynamic interactions, including but not limited to:
- Digitalis glycosides: Concomitant administration of digoxin and esmolol leads to an approximate 10% to 20% increase of digoxin blood levels at some time points. Digoxin does not affect esmolol pharmacokinetics. Both digoxin and beta blockers slow atrioventricular conduction and decrease the heart rate. Concomitant use increases the risk of bradycardia.
- Anticholinesterases: esmolol prolonged the duration of succinylcholine-induced neuromuscular blockade and moderately prolonged clinical duration and recovery index of mivacurium.
- Antihypertensive agents clonidine, guanfacine, or moxonidine: Beta blockers also increase the risk of clonidine-, guanfacine-, or moxonidine-withdrawal rebound hypertension. If, during concomitant use of a beta blocker, antihypertensive therapy needs to be interrupted or discontinued, discontinue the beta blocker first, and the discontinuation should be gradual.
- Calcium channel antagonists: In patients with depressed myocardial function, use of esmolol with cardiodepressant calcium channel antagonists (e.g., verapamil) can lead to fatal cardiac arrests.
- Sympathomimetic drugs: Sympathomimetic drugs having beta-adrenergic agonist activity will counteract effects of esmolol.
- Vasoconstrictive and positive inotropic agents: Because of the risk of reducing cardiac contractility in presence of high systemic vascular resistance, do not use esmolol to control tachycardia in patients receiving drugs that are vasoconstrictive and have positive inotropic effects, such as epinephrine, norepinephrine, and dopamine.
|FDAPregCat=C |useInPregnancyFDA=* Esmolol hydrochloride has been shown to produce increased fetal resorptions with minimal maternal toxicity in rabbits when given in doses approximately 8 times the maximum human maintenance dose (300 mcg/kg/min). There are no adequate and well-controlled studies in pregnant women. BREVIBLOC injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Teratogenicity studies in rats at intravenous dosages of esmolol hydrochloride up to 3000 mcg/kg/min (10 times the maximum human maintenance dosage) for 30 minutes daily produced no evidence of maternal toxicity, embryotoxicity or teratogenicity, while a dosage of 10,000 mcg/kg/min produced maternal toxicity and lethality. In rabbits, intravenous dosages up to 1000 mcg/kg/min for 30 minutes daily produced no evidence of maternal toxicity, embryotoxicity or teratogenicity, while 2500 mcg/kg/min produced minimal maternal toxicity and increased fetal resorptions.
|useInPregnancyAUS=(Description) |useInLaborDelivery=* Although there are no adequate and well-controlled studies in pregnant women, use of esmolol in the last trimester of pregnancy or during labor or delivery has been reported to cause fetal bradycardia, which continued after termination of drug infusion. Esmolol injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. |useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from esmolol, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. |useInPed=* The safety and effectiveness of esmolol in pediatric patients have not been established. |useInGeri=* Clinical studies of esmolol injection did not include sufficient numbers of subjects aged 65 and over to determine whether they responded differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should usually start at the low end of the dosing range, reflecting greater frequency of decreased renal or cardiac function and of concomitant disease or other drug therapy. |useInRenalImpair=* No dosage adjustment is required for esmolol in patients with renal impairment receiving a maintenance infusion of esmolol 150 mcg/kg for 4 hours. There is no information on the tolerability of maintenance infusions of esmolol using rates in excess of 150 mcg/kg or maintained longer than 4 hours. |useInHepaticImpair=* No special precautions are necessary in patients with hepatic impairment because esmolol is metabolized by red-blood cell esterases. |administration=Intravenous |monitoring=* After administration of the second dose of the alternative drug, monitor the patient's response and if satisfactory control is maintained for the first hour, discontinue the esmolol infusion.
- Monitor patients closely, especially if pretreatment blood pressure is low. In case of an unacceptable drop in blood pressure, reduce or stop esmolol injection. Decrease of dose or termination of infusion reverses hypotension, usually within 30 minutes.
- Monitor heart rate and rhythm in patients receiving esmolol.
- In patients with intraoperative and postoperative tachycardia and/or hypertension monitor vital signs closely and titrate smolol slowly in the treatment of patients whose blood pressure is primarily driven by vasoconstriction associated with hypothermia.
- Monitor serum electrolytes during therapy with esmolol.
- Monitor patients for signs of thyrotoxicosis when withdrawing beta blocking therapy.
|IVCompat=Esmolol was tested for compatibility with ten commonly used intravenous fluids at a final concentration of 10 mg esmolol hydrochloride per mL. was found to be compatible with the following solutions and was stable for at least 24 hours at controlled room temperature or under refrigeration:
- Dextrose (5%) Injection, USP
- Dextrose (5%) in Lactated Ringer’s Injection
- Dextrose (5%) in Ringer’s Injection
- Dextrose (5%) and Sodium Chloride (0.45%) Injection, USP
- Dextrose (5%) and Sodium Chloride (0.9%) Injection, USP
- Lactated Ringer’s Injection, USP
- Potassium Chloride (40 mEq/liter) in Dextrose (5%) Injection, USP
- Sodium Chloride (0.45%) Injection, USP
- Sodium Chloride (0.9%) Injection, USP
|overdose====Signs and Symptoms of Overdose===
Overdoses of esmolol can cause cardiac and central nervous system effects. These effects may precipitate severe signs, symptoms, sequelae, and complications (for example, severe cardiac and respiratory failure, including shock and coma), and may be fatal. Continuous monitoring of the patient is required.
Cardiac effects
- Bradycardia,atrioventricular block (1st -, 2nd -, 3rd degree), junctional rhythms, intraventricular conduction delays, decreased cardiac contractility,hypotension, cardiac failure (including cardiogenic shock), cardiac arrest/asystole, and pulseless electrical activity.
Central nervous system
- Respiratory depression,seizures, sleep and mood disturbances, fatigue, lethargy, and coma.
- In addition, bronchospasm, mesenteric ischemia, peripheral cyanosis, hyperkalemia, and hypoglycemia (especially in children) may occur.
Treatment Recommendations
- Because of its approximately 9-minute elimination half-life, the first step in the management of toxicity should be to discontinue the esmolol infusion. Then, based on the observed clinical effects, consider the following general measures.
Bradycardia
- Consider intravenous administration of atropine or another anticholinergic drug or cardiac pacing.
Cardiac Failure
- Consider intravenous administration of a diuretic or digitalis glycoside. In shock resulting from inadequate cardiac contractility, consider intravenous administration of dopamine, dobutamine, isoproterenol, or inamrinone. Glucagon has been reported to be useful.
Symptomatic hypotension
- Consider intravenous administration of fluids or vasopressor agents such as dopamine or norepinephrine.
Bronchospasm
- Consider intravenous administration of a beta 2 stimulating agent or a theophylline derivative.
Dilution Errors
- Massive accidental overdoses of esmolol have resulted from dilution errors. The use of esmolol premixed injection may reduce the potential for dilution errors. Some of these overdoses have been fatal while others resulted in permanent disability. Bolus doses in the range of 625 mg to 2.5 g (12.5-50 mg/kg) have been fatal. Patients have recovered completely from overdoses as high as 1.75 g given over one minute or doses of 7.5 g given over one hour for cardiovascular surgery. The patients who survived appear to be those whose circulation could be supported until the effects of esmolol resolved.
|drugBox={{Drugbox2 | Verifiedfields = changed | verifiedrevid = 412885041 | IUPAC_name = methyl (RS)-3-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}propanoate | image = Esmolol.JPG | tradename = | Drugs.com = Monograph | pregnancy_AU = C | pregnancy_US = C | pregnancy_category = | legal_status = | routes_of_administration = iv
| bioavailability = - | protein_bound = 60% | metabolism = Erythrocytic | elimination_half-life = 9 minutes | excretion = Renal
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 103598-03-4
| ATC_prefix = C07
| ATC_suffix = AB09
| PubChem = 59768
| DrugBank_Ref =
| DrugBank = DB00187
| ChemSpiderID_Ref =
| ChemSpiderID = 53916
| UNII_Ref =
| UNII = MDY902UXSR
| KEGG_Ref =
| KEGG = D07916
| ChEBI_Ref =
| ChEBI = 4856
| ChEMBL_Ref =
| ChEMBL = 768
| C=16 | H=25 | N=1 | O=4
| molecular_weight = 295.374 g/mol
| smiles = O=C(OC)CCc1ccc(OCC(O)CNC(C)C)cc1
| InChI = 1/C16H25NO4/c1-12(2)17-10-14(18)11-21-15-7-4-13(5-8-15)6-9-16(19)20-3/h4-5,7-8,12,14,17-18H,6,9-11H2,1-3H3
| InChIKey = AQNDDEOPVVGCPG-UHFFFAOYAE
| StdInChI_Ref =
| StdInChI = 1S/C16H25NO4/c1-12(2)17-10-14(18)11-21-15-7-4-13(5-8-15)6-9-16(19)20-3/h4-5,7-8,12,14,17-18H,6,9-11H2,1-3H3
| StdInChIKey_Ref =
| StdInChIKey = AQNDDEOPVVGCPG-UHFFFAOYSA-N
}}
|mechAction=Esmolol is a selective beta1 adrenergic receptor blocking agent with rapid onset, a very short duration of action, and no significant intrinsic sympathomimetic or membrane stabilizing activity at therapeutic dosages.
Its elimination half-life after intravenous infusion is approximately 9 minutes. esmolol inhibits the beta1 adrenergic receptors located chiefly in cardiac muscle, but this preferential effect is not absolute and at higher doses it begins to inhibit beta2 adrenergic receptors located chiefly in the bronchial and vascular musculature. |structure=Esmolol is a beta adrenergic receptor blocker with a very short duration of action (elimination half-life is approximately 9 minutes). Esmolol hydrochloride is:
(±)-Methyl p-[2-hydroxy-3-(isopropylamino) propoxy] hydrocinnamate hydrochloride and has the following structure:
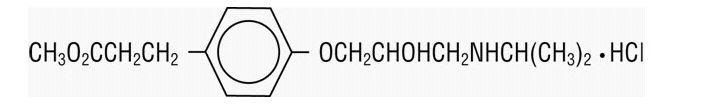
Esmolol hydrochloride has the empirical formula C16H26NO4Cl and a molecular weight of 331.8. It has one asymmetric center and exists as an enantiomeric pair.
Esmolol hydrochloride is a white to off-white crystalline powder. It is a relatively hydrophilic compound which is very soluble in water and freely soluble in alcohol. Its partition coefficient (octanol/water) at pH 7.0 is 0.42 compared to 17.0 for propranolol.
Esmolol Injection Dosage Forms
All esmolol presentations are clear, colorless to light yellow, sterile, nonpyrogenic, iso-osmotic solutions of esmolol hydrochloride in sodium chloride. The formulations for esmolol premixed injection, esmolol double strength injection, and esmolol injection are described in the table below:
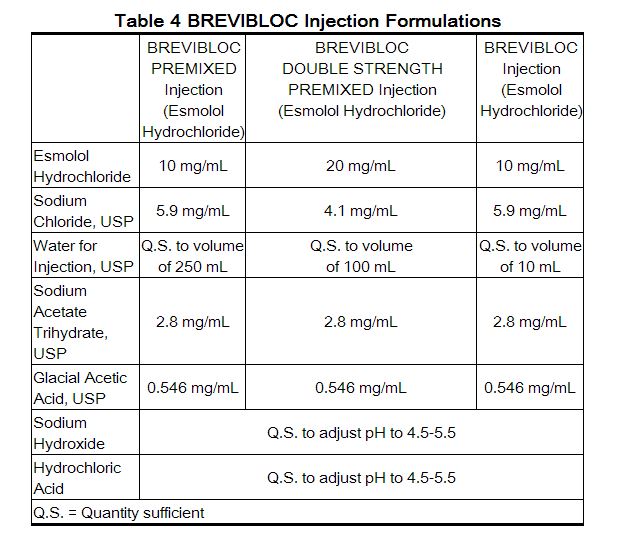
The calculated osmolarity of esmolol premixed injection and esmolol double strength injection is 312 mOsmol/L. The 250 mL and 100 mL bags are non-latex, non-PVC INTRAVIA bags with dual PVC ports. The INTRAVIA bags are manufactured from a specially designed multilayer plastic (PL 2408). Solutions in contact with the plastic container leach out certain chemical compounds from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. |PD=Clinical pharmacology studies in normal volunteers have confirmed the beta blocking activity of esmolol, showing reduction in heart rate at rest and during exercise, and attenuation of isoproterenol-induced increases in heart rate. Blood levels of esmolol have been shown to correlate with extent of beta blockade. After termination of infusion, substantial recovery from beta Blockade is observed in 10-20 minutes. The acid metabolite of esmolol exhibits negligible pharmacological activity.
In human electrophysiology studies, esmolol produced effects typical of a beta blocker: a decrease in the heart rate, increase in sinus cycle length, prolongation of the sinus node recovery time, prolongation of the AH interval during normal sinus rhythm and during atrial pacing, and an increase in antegrade Wenckebach cycle length.
In patients undergoing radionuclide angiography, esmolol, at dosages of 200 mcg/kg/min, produced reductions in heart rate, systolic blood pressure, rate pressure product, left and right ventricular ejection fraction and cardiac index at rest, which were similar in magnitude to those produced by intravenous propranolol (4 mg). During exercise, esmolol produced reductions in heart rate, rate pressure product and cardiac index which were also similar to those produced by propranolol, but esmolol produced a significantly larger fall in systolic blood pressure. In patients undergoing cardiac catheterization, the maximum therapeutic dose of 300 mcg/kg/min of esmolol produced similar effects and, in addition, there were small, clinically insignificant increases in the left ventricular end diastolic pressure and pulmonary capillary wedge pressure. At 30 minutes after the discontinuation of esmolol infusion, all of the hemodynamic parameters had returned to pretreatment levels.
The relative cardioselectivity of esmolol was demonstrated in 10 mildly asthmatic patients. Infusions of esmolol 100, 200 and 300 mcg/kg/min produced no significant increases in specific airway resistance compared to placebo. At 300 mcg/kg/min, esmolol produced slightly enhanced bronchomotor sensitivity to dry air stimulus. These effects were not clinically significant, and esmolol was well tolerated by all patients. Six of the patients also received intravenous propranolol, and at a dosage of 1 mg, two experienced significant, symptomatic bronchospasm requiring bronchodilator treatment. One otherpropranolol-treated patient also experienced dry air-induced bronchospasm. No adverse pulmonary effects were observed in patients with COPD who received therapeutic dosages of esmolol for treatment of supraventricular tachycardia (51 patients) or in perioperative settings (32 patients). |PK=Esmolol is rapidly metabolized by hydrolysis of the ester linkage, chiefly by the esterases in the cytosol of red blood cells and not by plasma cholinesterases or red cell membrane acetylcholinesterase. Total body clearance in man was found to be about 20 L/kg/hr, which is greater than cardiac output; thus the metabolism of esmolol is not limited by the rate of blood flow to metabolizing tissues such as the liver or affected by hepatic or renal blood flow. Esmolol has a rapid distribution half-life of about 2 minutes and an elimination half-life of about 9 minutes.
Using an appropriate loading dose, steady-state blood levels of esmolol for dosages from 50-300 mcg/kg/min are obtained within five minutes. Steady-state is reached in about 30 minutes without the loading dose. Steady-state blood levels of esmolol increase linearly over this dosage range and elimination kinetics are dose-independent over this range. Steady-state blood levels are maintained during infusion but decrease rapidly after termination of the infusion. Because of its short half-life, blood levels of esmolol can be rapidly altered by increasing or decreasing the infusion rate and rapidly eliminated by discontinuing the infusion.
Consistent with the high rate of blood-based metabolism of esmolol, less than 2% of the drug is excreted unchanged in the urine. Within 24 hours of the end of infusion, the acid metabolite of esmolol in urine accounts for approximately 73-88% of the dosage.
Metabolism of esmolol results in the formation of the corresponding free acid and methanol. The acid metabolite has been shown in animals to have negligible activity and in normal volunteers its blood levels do not correspond to the level of beta blockade. The acid metabolite has an elimination half-life of about 3.7 hours and is excreted in the urine with a clearance approximately equivalent to the glomerular filtration rate. After a 4 hour maintenance infusion of 150 mcg/kg, the plasma concentrations of esmolol are similar in subjects with normal renal function and in patients with ESRD on dialysis. The half-life of the acid metabolite of esmolol, which is primarily excreted unchanged by the kidney, is increased about 12-fold to 48 hours in patients with ESRD. The peak concentrations of the acid metabolite are doubled in ESRD.
Methanol blood levels, monitored in subjects receiving esmolol for up to 6 hours at 300 mcg/kg/min and 24 hours at 150 mcg/kg/min, approximated endogenous levels and were less than 2% of levels usually associated with methanol toxicity.
Esmolol has been shown to be 55% bound to human plasma protein, while the acid metabolite is only 10% bound. |nonClinToxic=Because of its short term usage no carcinogenicity, mutagenicity, or reproductive performance studies have been conducted with esmolol. |clinicalStudies======Supraventricular Tachycardia=====
In two multicenter, randomized, double-blind, controlled comparisons of esmolol injection with placebo and propranolol, maintenance doses of 50 to 300 mcg/kg/min of esmolol were found to be more effective than placebo and about as effective as propranolol, 3-6 mg given by bolus injections, in the treatment of supraventricular tachycardia, principally atrial fibrillation and atrial flutter. The majority of these patients developed their arrhythmias postoperatively. About 60-70% of the patients treated with esmolol developed either a 20% reduction in heart rate, a decrease in heart rate to less than 100 bpm, or, rarely, conversion to normal sinus rhythm and about 95% of these patients did so at a dosage of 200 mcg/kg/min or less. The average effective dosage of esmolol was approximately 100 mcg/kg/min in the two studies. Other multicenter baseline-controlled studies gave similar results. In the comparison with propranolol, about 50% of patients in both the esmolol and propranolol groups were on concomitant digoxin. Response rates were slightly higher with both beta blockers in the digoxin-treated patients.
In all studies significant decreases of blood pressure occurred in 20-50% of patients, identified either as adverse reaction reports by investigators, or by observation of systolic pressure less than 90 mmHg or diastolic pressure less than 50 mmHg. The hypotension was symptomatic (mainly hyperhidrosis or dizziness) in about 12% of patients, and therapy was discontinued in about 11% of patients, about half of whom were symptomatic. Hypotension was more common with esmolol (53%) than with propranolol (17%). The hypotension was rapidly reversible with decreased infusion rate or after discontinuation of therapy with esmolol. For both esmolol and propranolol, hypotension was reported less frequently in patients receiving concomitant digoxin. |howSupplied=* BREVIBLOC PREMIXED Injection NDC 10019-055-61, 2500 mg / 250 mL (10 mg/mL) Ready-to-use INTRAVIA Bags
- BREVIBLOC DOUBLE STRENGTH PREMIXED Injection
NDC 10019-075-87, 2000 mg / 100 mL (20 mg/mL) Ready-to-use INTRAVIA Bags
- BREVIBLOC Injection
NDC 10019-115-01, 100 mg / 10 mL (10 mg/mL) Ready-to-use Vials, Package of 25 |storage=* Store at 25˚C (77˚F). Excursions permitted to 15˚-30˚C (59˚-86˚F). Protect from freezing. Avoid excessive heat.
- Each bag contains no preservative. Once drug has been withdrawn from ready-to-use bag, the bag should be used within 24 hours, with any unused portion discarded.
- Do not use plastic containers in series connections. Such use could result in an embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
- Do not remove unit from overwrap until ready to use. Do not use if overwrap has been previously opened or damaged. The overwrap is a moisture barrier. The inner bag maintains sterility of the solution. Tear overwrap at notch and remove premixed bag. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
- Check for minute leaks by squeezing the inner bag firmly. If leaks are found, discard solution, as sterility may be impaired. Do not use unless the solution is clear (colorless to light yellow) and the seal is intact.
|packLabel=


|fdaPatientInfo=Physicians should inform patients of the risks associated with BREVIBLOC injection:
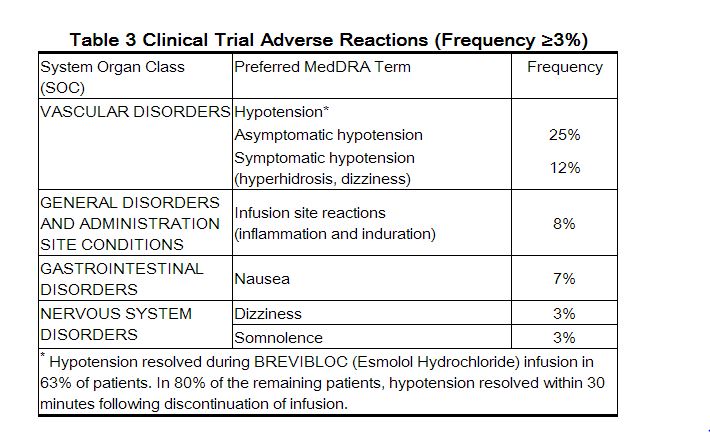
- The most common adverse reactions are symptomatic hypotension (hyperhidrosis, dizziness), and asymptomatic hypotension.
|alcohol=Alcohol-Esmolol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=* Brevibloc
- Esmolol HCl
|lookAlike=* Brevibloc - Brevital |nlmPatientInfo=(Link to patient information page) |drugShortage=Drug Shortage }}
- ↑ January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE; et al. (2014). "2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society". J Am Coll Cardiol. doi:10.1016/j.jacc.2014.03.022. PMID 24685669.
- ↑ Mooss AN, Hilleman DE, Mohiuddin SM, Hunter CB (1994). "Safety of esmolol in patients with acute myocardial infarction treated with thrombolytic therapy who had relative contraindications to beta-blocker therapy". Ann Pharmacother. 28 (6): 701–3. PMID 7919552.
- ↑ Johansen JW, Flaishon R, Sebel PS (1997). "Esmolol reduces anesthetic requirement for skin incision during propofol/nitrous oxide/morphine anesthesia". Anesthesiology. 86 (2): 364–71. PMID 9054254.
- ↑ van den Broek WW, Leentjens AF, Mulder PG, Kusuma A, Bruijn JA (1999). "Low-dose esmolol bolus reduces seizure duration during electroconvulsive therapy: a double-blind, placebo-controlled study". Br J Anaesth. 83 (2): 271–4. PMID 10618942.
- ↑ Mehlhorn U, Sauer H, Kuhn-Régnier F, Südkamp M, Dhein S, Eberhardt F; et al. (1999). "Myocardial beta-blockade as an alternative to cardioplegic arrest during coronary artery surgery". Cardiovasc Surg. 7 (5): 549–57. PMID 10499899.
- ↑ Zakowski M, Kaufman B, Berguson P, Tissot M, Yarmush L, Turndorf H (1989). "Esmolol use during resection of pheochromocytoma: report of three cases". Anesthesiology. 70 (5): 875–7. PMID 2566290.
- ↑ Duggal J, Singh S, Kuchinic P, Butler P, Arora R (2006). "Utility of esmolol in thyroid crisis". Can J Clin Pharmacol. 13 (3): e292–5. PMID 17127774.