Propranolol detailed information
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | oral, iv |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 26% |
| Metabolism | hepatic (extensive) |
| Elimination half-life | 4-5 hours |
| Excretion | renal <1% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
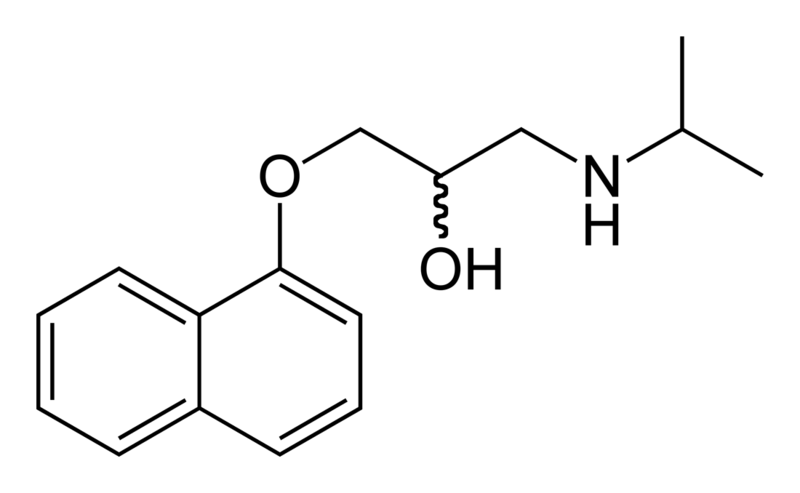
| Formula | C16H21NO2 |
| Molar mass | 259.34 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
For patient information, click here
Propranolol (INN) (IPA: Template:IPA) is a non-selective beta blocker mainly used in the treatment of hypertension. It was the first successful beta blocker developed. Propranolol is available in generic form as propranolol hydrochloride, as well as an AstraZeneca product under the trade names Inderal, Inderal LA, Avlocardyl, Avlocardyl retard, Dociton, Inderalici, Sumial (depending on marketplace and release rate). It is also marketed by Wyeth.
History and development
Scottish scientist James W. Black successfully developed propranolol in the late 1950s. He was awarded the Nobel Prize in Medicine for this discovery in 1988.
Propranolol developed from the early β-adrenergic antagonists dichloroisoprenaline and pronethalol. The key structural modification, which was carried through to essentially all subsequent beta blockers, was the insertion of an oxymethylene bridge into the arylethanolamine structure of pronethalol thus greatly increasing the potency of the compound. This also apparently eliminated the carcinogenicity found with pronethalol in animal models.
Pharmacology
Propranolol is a non-selective beta blocker, that is, it blocks the action of epinephrine on both β1- and β2-adrenergic receptors. It has little intrinsic sympathomimetic activity (ISA) but has strong membrane stabilizing activity (only at high blood concentrations, eg overdosage).
Pharmacokinetics
Propranolol is rapidly and completely absorbed, with peak plasma levels achieved approximately 1–3 hours after ingestion. Co-administration with food appears to enhance bioavailability. Despite complete absorption, propranolol has a variable bioavailability due to extensive first-pass metabolism. Hepatic impairment will therefore increase its bioavailability. The main metabolite 4-hydroxypropranolol, with a longer half-life (5.2–7.5 hours) than the parent compound (3–4 hours), is also pharmacologically active.
Propranolol is a highly lipophilic drug achieving high concentrations in the brain. The duration of action of a single oral dose is longer than the half-life indicates and may be up to 12 hours, if the single dose is high enough (e.g., 80 mg). Effective plasma concentrations are between 10–100 ng/mL.
Toxic levels are associated with plasma concentrations above 2000 ng/ml
Clinical use
Indications
Propranolol is indicated for the management of various conditions including:[1]
- Hypertension
- Angina pectoris
- Tachyarrhythmias
- Myocardial infarction
- Control of tachycardia/tremor associated with anxiety and hyperthyroidism
- Essential tremor
- Migraine prophylaxis
- Tetralogy of Fallot
- Phaeochromocytoma (along with α blocker)
- Post Traumatic Stress Disorder (experimental)
While once first-line treatment for hypertension, the role for beta-blockers was downgraded in June 2006 in the United Kingdom to fourth-line as they perform less well than other drugs, particularly in the elderly, and evidence is increasing that the most frequently used beta-blockers at usual doses carry an unacceptable risk of provoking type 2 diabetes. [2]
Propranolol is also used to lower portal vein pressure in portal hypertension and prevent oesophageal variceal bleeding.
Propranolol is often used by musicians and other performers to prevent stage fright.
Propranolol is currently being investigated as a potential treatment for post-traumatic stress disorder. [3][4][5]
Precautions/contraindications
Propranolol should be used with caution in patients with:[1]
- Diabetes mellitus or hyperthyroidism, since signs and symptoms of hypoglycaemia may be masked
- Peripheral vascular disease and Raynaud's syndrome, which may be exacerbated
- Phaeochromocytoma, as hypertension may be aggravated without prior alpha blocker therapy
- Myasthenia gravis, may be worsened
- Other drugs with bradycardic effects
Propranolol is contraindicated in patients with:[1]
- Reversible airways disease, particularly asthma or chronic obstructive pulmonary disease (COPD)
- Bradycardia (<50 beats/minute)
- Sick sinus syndrome
- Atrioventricular block (second or third degree)
- Shock
- Severe hypotension
- Uncontrolled congestive heart failure
- Cocaine toxicity [per American Heart Association guidelines, 2005]
Adverse effects
Adverse drug reactions (ADRs) associated with propranolol therapy are similar to other lipophilic beta blockers (see beta blocker).
Pregnancy and Lactation
Propranolol, like other beta blockers, is classified as Pregnancy category C in the United States and ADEC Category C in Australia. Beta-blocking agents in general reduce perfusion of the placenta which may lead to adverse outcomes for the neonate, including pulmonary or cardiac complications, or premature birth. The newborn may experience additional adverse effects such as hypoglycemia and bradycardia.
Most beta-blocking agents appear in the milk of lactating women. This is especially the case for a lipophilic drug like propranolol. Breastfeeding is not recommended in patients receiving propranolol therapy.
Interactions
Beta blockers, including propranolol, have an additive effect with other drugs which decrease blood pressure, or which decrease cardiac contractility or conductivity. Clinically-significant interactions particularly occur with:[1]
- verapamil
- epinephrine
- β2-adrenergic receptor agonists
- clonidine
- ergot alkaloids
- isoprenaline
- non-steroidal anti-inflammatory drugs
- quinidine
- cimetidine
- lidocaine
- phenobarbital
- rifampicin
Dosage
The usual maintenance dose ranges for oral propranolol therapy vary by indication:
- Hypertension, angina, essential tremor
- 120–320 mg daily in divided doses.
- Sustained-release formulations are available in some markets.
- Tachyarrhythmia, anxiety, hyperthyroidism
- 10–40 mg 3–4 times daily
Intravenous (IV) propranolol may be used in acute arrhythmia or thyrotoxic crisis.[6]
Research into role against malaria
Propranolol along with a number of other membrane-acting drugs have been investigated for possible effects on P. falciparum and so the treatment of malaria. In vitro positive effects until recently had not been matched by useful in vivo anti-parasite activity against Plasmodium vinckei,[7] or P. yoelii nigeriensis.[8] However a single study from 2006 has suggested that propranolol may reduce the dosages required for existing drugs to be effective against P. falciparum by 5- to 10-fold, suggesting a role for combination therapies.[9]
Media
- Propranolol as an experimental treatment for Post Traumatic Stress Disorder was a subject of the television show Boston Legal, Season 3, Episode 14, Selling Sickness.
- Propanonol was used by one of the criminals in the TV show CSI: NY episode titled "Some buried bones" , Season 3 Episode 15 (2004-02-17).
- Propranolol was referenced on the Late Show With David Letterman on May 4, 2007 in a mock-advertisement.
References
- ↑ 1.0 1.1 1.2 1.3 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- ↑ Sheetal Ladva (28/06/2006). "NICE and BHS launch updated hypertension guideline". National Institute for Health and Clinical Excellence. Check date values in:
|date=(help) - ↑ "Doctors test a drug to ease traumatic memories - Mental Health - MSNBC.com".
- ↑ Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK (2007). "Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder". doi:10.1016/j.jpsychires.2007.05.006. PMID 17588604.
- ↑ "Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder".
- ↑ *Joint Formulary Committee. British National Formulary, 47th edition. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2004.
- ↑ Ohnishi S, Sadanaga K, Katsuoka M, Weidanz W (1990). "Effects of membrane acting-drugs on plasmodium species and sickle cell erythrocytes". Mol Cell Biochem. 91 (1–2): 159–65. PMID 2695829.
- ↑ Singh N, Puri S (2000). "Interaction between chloroquine and diverse pharmacological agents in chloroquine resistant Plasmodium yoelii nigeriensis". Acta Trop. 77 (2): 185–93. PMID 11080509.
- ↑ Murphy S, Harrison T, Hamm H, Lomasney J, Mohandas N, Haldar K (2006). "Erythrocyte G protein as a novel target for malarial chemotherapy". PLoS Med. 3 (12): e528. PMID 17194200. Unknown parameter
|month=ignored (help)
External links
- Beta-adrenergic blocking agents (systemic) – information from USP DI Advice for the Patient
- Historical Remarks about Propranolol and its inventor
- Scientific American Interview with James McGaugh
- CBS NEWS 60 Minutes: A Pill To Forget
- Pages with script errors
- CS1 errors: dates
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Antiarrhythmic agents
- Anxiolytics
- Beta blockers
- Drugs