Spironolactone detailed information
 | |
| Clinical data | |
|---|---|
| Synonyms | Aldactone Spirotone Spirolactone |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 10 minutes |
| Excretion | Urine, bile |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
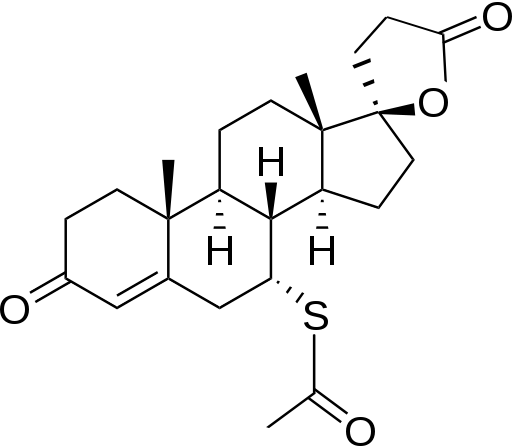
| Formula | C24H32O4S |
| Molar mass | 416.574 g/mol |
| 3D model (JSmol) | |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
For patient information, click here
Spironolactone (marketed under the trade names Aldactone, Novo-Spiroton, Spiractin, Spirotone, Verospiron or Berlactone) is a diuretic and is used as an antiandrogen.
It is a synthetic 17-lactone drug which is a renal competitive aldosterone antagonist in a class of pharmaceuticals called potassium-sparing diuretics, used primarily to treat ascites in patients with liver disease, low-renin hypertension, hypokalemia, and Conn's syndrome as well as high blood pressure. On its own, spironolactone is only a weak diuretic, but it can be combined with other diuretics. About one person in one hundred with hypertension has elevated levels of aldosterone; in these persons the antihypertensive effect of spironolactone may exceed that of complex combined regimens of other antihypertensives. Due to its anti-androgen effect, it can also be used to treat hirsutism, and is a common component in hormone therapy for male-to-female transsexual and transgendered people. It is also used for treating hair loss and acne in women and can be used as a topical medication for treatment of male hairloss.
Mechanism of action
Spironolactone inhibits the effect of aldosterone by competing for intracellular aldosterone receptor in the distal tubule cells. This increases the secretion of water and sodium, while decreasing the excretion of potassium. Spironolactone has a fairly slow onset of action, taking several days to develop and similarly the effect diminishes slowly. Spironolactone has anti-androgen activity by binding to the androgen receptor and thus preventing it to interact with dihydrotestosterone.[1]
Pharmacokinetics
Spironolactone is fairly rapidly absorbed from the gastrointestinal tract. It is also rapidly metabolised and bound in plasma proteins. Many of its metabolites are also active and one of them, canrenone as potassium canrenoate, is used parenterally when rapid effect is needed. Spironolactone's half-life is 85 minutes, but canrenone's half-life is 10 to 35 hours, depending on the dose. The main elimination route is in the urine and some also in the bile.
Mortality and morbidity benefit in severe heart failure
Spironolactone was shown to have a significant mortality and morbidity benefit in the Randomized Aldactone Evaluation Study (RALES), which studied people with severe congestive heart failure (New York Heart Association functional class III or IV).[2] Patients in the study arm of the trial (those receiving spironolactone) had a relative risk of death (when compared to the placebo group) equal to 0.70 or a 30% relative risk reduction. Patients in the study arm also had significantly less symptoms of CHF and were hospitalized less frequently.
Adverse effects and interactions
Following the publication of the RALES study, there was an increase in the incidence of hyperkalemia, particularly among those patients treated concomitantly with ACE inhibitors[3]. Spironolactone is associated with an increased risk of bleeding from the stomach and duodenum, but a causal relationship between the two has not been established.[4] Since it also affects steroid receptors elsewhere in the body, it can cause gynecomastia, menstrual irregularities and testicular atrophy. Other side effects include ataxia, erectile dysfunction, drowsiness and rashes. A carcinogenic effect has been demonstrated in rats. Spironolactone has been shown to be immunosuppressive in the treatment of sarcoidosis.[5]
People using this drug should avoid salt substitutes containing potassium.[6]
Carcinogenicity
Studies of spironolactone and the related compound potassium canrenoate (which, like spironolactone, metabolizes to canrenone) in rats for one to two year periods show an increase in carcinogenesis in the thyroid gland, testes, liver, breasts, and myelocytic leukocytes. Mammalian cells, depending on the presence of metabolic activation, show mixed results for mutagenicity in vitro.[7] In light of this research, Sandoz has recommended that unnecessary use of spironolactone be avoided.
See also
References
- ↑ Berardesca, E (1988). "Topical spironolactone inhibits dihydrotestosterone receptors in human sebaceous glands: an autoradiographic study in subjects with acne vulgaris". Int J Tissue React. 10 (2): 115–119. PMID 2972662. Text " publisher " ignored (help); Unknown parameter
|coauthors=ignored (help) - ↑ Pitt B, Zannad F, Remme W, Cody R, Castaigne A, Perez A, Palensky J, Wittes J (1999). "The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators". N Engl J Med. 341 (10): 709–17. PMID 10471456.
- ↑ Juurlink DN, Mamdani MM, Lee DS; et al. (2004). "Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study". The New England Journal of Medicine. 351 (6): 543–51. doi:10.1056/NEJMoa040135. PMID 15295047. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Verhamme KMC, Mosis G, Dieleman JP; et al. (2006). "Spironolactone and risk of upper gastrointestinal events: population based case-control study". Brit Med J. 333 (7563): 330&ndash, 3. doi:10.1136/bmj.38883.479549.2F.
- ↑ Wandelt-Freerksen E. (1977). "Aldactone in the treatment of sarcoidosis of the lungs". JZ Erkr Atmungsorgane. 149(1): 156–9. PMID 607621.
- ↑ "Advisory Statement" (pdf). Klinge Chemicals / LoSalt.
- ↑ "Spironolactone RX Monograph" (html). Sandoz Inc.
External links
de:Spironolacton hr:Spironolakton hu:Spironolakton nl:Spironolacton
- Pages with script errors
- Pages with citations using unnamed parameters
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- Pages using citations with accessdate and no URL
- CS1 maint: Explicit use of et al.
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Aldosterone antagonists
- Antiandrogens
- Lactones
- Hair loss
- Drugs
- Endocrinology