Doripenem microbiology: Difference between revisions
Gerald Chi (talk | contribs) mNo edit summary |
|||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Doripenem}} | {{Doripenem}} | ||
{{CMG}} | {{CMG}}; {{AE}} {{SS}} | ||
==Microbiology== | ==Microbiology== | ||
===Mechanism of Action=== | ===Mechanism of Action=== | ||
Doripenem belongs to the carbapenem class of antimicrobials. Doripenem exerts its bactericidal activity by inhibiting bacterial cell wall biosynthesis. Doripenem inactivates multiple essential penicillin-binding | Doripenem belongs to the [[carbapenem]] class of antimicrobials. Doripenem exerts its bactericidal activity by inhibiting bacterial cell wall biosynthesis. Doripenem inactivates multiple essential [[penicillin-binding protein]]s (PBPs) resulting in inhibition of cell wall synthesis with subsequent cell death. In [[E. coli]] and [[P. aeruginosa]], doripenem binds to PBP 2, which is involved in the maintenance of cell shape, as well as to PBPs 3 and 4. | ||
===Mechanism(s) of Resistance=== | ===Mechanism(s) of Resistance=== | ||
Bacterial resistance mechanisms that affect doripenem include drug inactivation by carbapenem-hydrolyzing enzymes, mutant or acquired PBPs, decreased outer membrane permeability and active efflux. Doripenem is stable to hydrolysis by most beta-lactamases, including | Bacterial resistance mechanisms that affect doripenem include drug inactivation by [[carbapenem]]-hydrolyzing enzymes, mutant or acquired PBPs, decreased outer membrane permeability and active efflux. Doripenem is stable to hydrolysis by most [[beta-lactamases]], including [[penicillinase]]s and cephalosporinases produced by Gram-positive and Gram-negative bacteria, with the exception of [[carbapenem]] hydrolyzing [[beta-lactamases]]. Although cross-resistance may occur, some isolates resistant to other [[carbapenem]]s may be susceptible to doripenem. | ||
====Interaction with Other Antimicrobials==== | ====Interaction with Other Antimicrobials==== | ||
In vitro synergy tests with doripenem show doripenem has little potential to antagonize or be antagonized by other antibiotics (e.g., levofloxacin, amikacin, trimethoprim-sulfamethoxazole, daptomycin, linezolid, and vancomycin | In vitro synergy tests with doripenem show doripenem has little potential to antagonize or be antagonized by other antibiotics (e.g., [[levofloxacin]], [[amikacin]], [[trimethoprim]]-[[sulfamethoxazole]], [[daptomycin]], [[linezolid]], and [[vancomycin]]. | ||
Doripenem has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections. | Doripenem has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections. | ||
| Line 17: | Line 20: | ||
=====Facultative Gram-negative microorganisms===== | =====Facultative Gram-negative microorganisms===== | ||
*Acinetobacter baumannii | * [[Acinetobacter baumannii]] | ||
*Escherichia coli | * [[Escherichia coli]] | ||
*Klebsiella pneumoniae | * [[Klebsiella pneumoniae]] | ||
*Proteus mirabilis | * [[Proteus mirabilis]] | ||
*Pseudomonas aeruginosa | * [[Pseudomonas aeruginosa]] | ||
====Facultative Gram-positive microorganisms===== | |||
*Streptococcus constellatus | * [[Streptococcus constellatus]] | ||
*Streptococcus intermedius | * [[Streptococcus intermedius]] | ||
=====Anaerobic microorganisms==== | =====Anaerobic microorganisms===== | ||
*Bacteroides caccae | * [[Bacteroides]] caccae | ||
*Bacteroides fragilis | * [[Bacteroides]] fragilis]] | ||
*Bacteroides | * [[Bacteroides]] thetaitaomicron | ||
*Bacteroides uniformis | * [[Bacteroides]] uniformis | ||
*Bacteroides vulgatus | * [[Bacteroides]] vulgatus | ||
*Peptostreptococcus micros | * [[Peptostreptococcus micros]] | ||
At least 90 percent of the following microorganisms exhibit an in vitro minimal inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for doripenem of organisms of the same type shown in Table | At least 90 percent of the following microorganisms exhibit an in vitro minimal inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for doripenem of organisms of the same type shown in Table below. The safety and efficacy of doripenem in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled clinical trials. | ||
=====Facultative Gram-positive microorganisms===== | =====Facultative Gram-positive microorganisms===== | ||
Staphylococcus aureus (methicillin-susceptible isolates only) | * [[Staphylococcus aureus]] ([[methicillin]]-susceptible isolates only) | ||
Streptococcus agalactiae | * [[Streptococcus agalactiae]] | ||
Streptococcus pyogenes | * [[Streptococcus pyogenes]] | ||
=====Facultative Gram-negative microorganisms===== | =====Facultative Gram-negative microorganisms===== | ||
*Citrobacter freundii | * [[Citrobacter freundii]] | ||
*Enterobacter cloacae | * [[Enterobacter cloacae]] | ||
*Enterobacter aerogenes | * [[Enterobacter aerogenes]] | ||
*Klebsiella oxytoca | * [[Klebsiella oxytoca]] | ||
*Morganella morganii | * [[Morganella morganii]] | ||
*Serratia marcescens | * [[Serratia marcescens]] | ||
====Susceptibility Test Methods==== | ====Susceptibility Test Methods==== | ||
| Line 78: | Line 81: | ||
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (1,3) (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of doripenem powder. The MIC values should be interpreted according to the criteria provided in Table below. | Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (1,3) (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of doripenem powder. The MIC values should be interpreted according to the criteria provided in Table below. | ||
Diffusion Techniques | =====Diffusion Techniques===== | ||
Quantitative methods that require measurement of zone diameters provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure (2,3) requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 10 µg of doripenem to test the susceptibility of microorganisms to doripenem. Results should be interpreted according to the criteria in Table below. | Quantitative methods that require measurement of zone diameters provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure (2,3) requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 10 µg of doripenem to test the susceptibility of microorganisms to doripenem. Results should be interpreted according to the criteria in Table below. | ||
Anaerobic Techniques | =====Anaerobic Techniques===== | ||
For anaerobic bacteria, the susceptibility to doripenem as MICs should be determined by standardized test methods (4). The MIC values obtained should be interpreted according to the criteria in Table below. | For anaerobic bacteria, the susceptibility to doripenem as MICs should be determined by standardized test methods (4). The MIC values obtained should be interpreted according to the criteria in Table below. | ||
{| | {| | ||
|- | |- | ||
Latest revision as of 22:10, 5 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Microbiology
Mechanism of Action
Doripenem belongs to the carbapenem class of antimicrobials. Doripenem exerts its bactericidal activity by inhibiting bacterial cell wall biosynthesis. Doripenem inactivates multiple essential penicillin-binding proteins (PBPs) resulting in inhibition of cell wall synthesis with subsequent cell death. In E. coli and P. aeruginosa, doripenem binds to PBP 2, which is involved in the maintenance of cell shape, as well as to PBPs 3 and 4.
Mechanism(s) of Resistance
Bacterial resistance mechanisms that affect doripenem include drug inactivation by carbapenem-hydrolyzing enzymes, mutant or acquired PBPs, decreased outer membrane permeability and active efflux. Doripenem is stable to hydrolysis by most beta-lactamases, including penicillinases and cephalosporinases produced by Gram-positive and Gram-negative bacteria, with the exception of carbapenem hydrolyzing beta-lactamases. Although cross-resistance may occur, some isolates resistant to other carbapenems may be susceptible to doripenem.
Interaction with Other Antimicrobials
In vitro synergy tests with doripenem show doripenem has little potential to antagonize or be antagonized by other antibiotics (e.g., levofloxacin, amikacin, trimethoprim-sulfamethoxazole, daptomycin, linezolid, and vancomycin.
Doripenem has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections.
Facultative Gram-negative microorganisms
Facultative Gram-positive microorganisms=
Anaerobic microorganisms
- Bacteroides caccae
- Bacteroides fragilis]]
- Bacteroides thetaitaomicron
- Bacteroides uniformis
- Bacteroides vulgatus
At least 90 percent of the following microorganisms exhibit an in vitro minimal inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for doripenem of organisms of the same type shown in Table below. The safety and efficacy of doripenem in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled clinical trials.
Facultative Gram-positive microorganisms
- Staphylococcus aureus (methicillin-susceptible isolates only)
Facultative Gram-negative microorganisms
Susceptibility Test Methods
When available, the clinical microbiology laboratory should provide the results of in vitro susceptibility test results for antimicrobial drugs used in local hospitals and practice areas to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting the most effective antimicrobial. Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (1,3) (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of doripenem powder. The MIC values should be interpreted according to the criteria provided in Table below.
Diffusion Techniques
Quantitative methods that require measurement of zone diameters provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure (2,3) requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 10 µg of doripenem to test the susceptibility of microorganisms to doripenem. Results should be interpreted according to the criteria in Table below.
Anaerobic Techniques
For anaerobic bacteria, the susceptibility to doripenem as MICs should be determined by standardized test methods (4). The MIC values obtained should be interpreted according to the criteria in Table below.
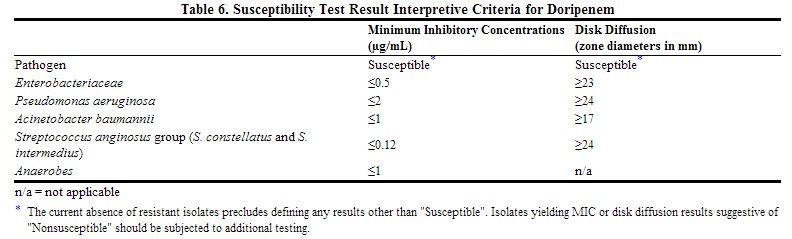 |
A report of Susceptible indicates that the antimicrobial is likely to inhibit growth of the pathogen if the antimicrobial compound in the blood reaches the concentrations usually achievable.
Quality Control
Standardized susceptibility test procedures require the use of laboratory control microorganisms to monitor the performance of the supplies and reagents used in the assay, and the techniques of the individuals performing the test. Standard doripenem powder should provide the MIC values provided in Table 7. For the diffusion techniques using a 10 µg doripenem disk, the criteria noted in Table 7 should be achieved.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022106s014lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.