Doripenem dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Dosage and Administration
Recommended Dosage
The recommended dosage of DORIBAX® is 500 mg administered every 8 hours by intravenous infusion over one hour in patients ≥18 years of age. The recommended dosage and administration by infection is described in Table below:
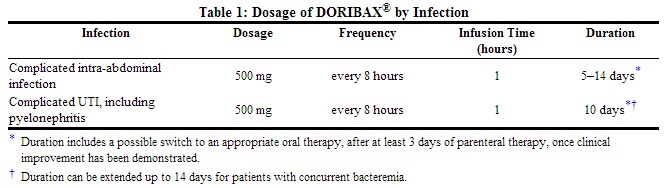 |
Patients With Renal Impairment
 |
The following formula may be used to estimate CrCl. The serum creatinine used in the formula should represent a steady state of renal function.
 |
DORIBAX® is hemodialyzable; however, there is insufficient information to make dose adjustment recommendations in patients on hemodialysis.
Preparation of Solutions
DORIBAX® does not contain a bacteriostatic preservative. Aseptic technique must be followed in preparation of the infusion solution.
To prepare DORIBAX infusions in Baxter Minibag Plus™ infusion bags consult the infusion bag manufacturer's instructions.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to use whenever solution and container permit. DORIBAX infusions range from clear, colorless solutions to solutions that are clear and slightly yellow. Variations in color within this range do not affect the potency of the product.
Preparation of 500 mg DORIBAX® dose using the 500 mg vial
Constitute the 500 mg vial with 10 mL of sterile water for injection or 0.9% sodium chloride injection (normal saline) and gently shake to form a suspension. The resultant concentration is approximately 50 mg/mL. CAUTION: THE CONSTITUTED SUSPENSION IS NOT FOR DIRECT INJECTION. Withdraw the suspension using a syringe with a 21 gauge needle and add it to an infusion bag containing 100 mL of normal saline or 5% dextrose; gently shake until clear. The final infusion solution concentration is approximately 4.5 mg/mL.
Preparation of 250 mg DORIBAX® dose using the 250 mg vial
Constitute the 250 mg vial with 10 mL of sterile water for injection or 0.9% sodium chloride injection (normal saline) and gently shake to form a suspension. The resultant concentration is approximately 25 mg/mL. CAUTION: THE CONSTITUTED SUSPENSION IS NOT FOR DIRECT INJECTION. Withdraw the suspension using a syringe with a 21 gauge needle and add it to an infusion bag containing either 50 or 100 mL of normal saline or 5% dextrose; gently shake until clear. The final infusion solution concentration is approximately 4.2 mg/mL (50 mL infusion bag) or approximately 2.3 mg/mL (100 mL infusion bag).
Preparation of 250 mg DORIBAX® dose using the 500 mg vial
Constitute the 500 mg vial with 10 mL of sterile water for injection or 0.9% sodium chloride injection (normal saline) and gently shake to form a suspension. The resultant concentration is approximately 50 mg/mL. CAUTION: THE CONSTITUTED SUSPENSION IS NOT FOR DIRECT INJECTION. Withdraw the suspension using a syringe with a 21 gauge needle and add it to an infusion bag containing 100 mL of normal saline or 5% dextrose; gently shake until clear. Remove 55 mL of this solution from the bag and discard. Infuse the remaining solution, which contains 250 mg (approximately 4.5 mg/mL).
Compatibility
The compatibility of DORIBAX® with other drugs has not been established. DORIBAX® should not be mixed with or physically added to solutions containing other drugs.
Storage of Constituted Solutions
Upon constitution with sterile water for injection or 0.9% sodium chloride (normal saline) injection, DORIBAX suspension in the vial may be held for 1-hour prior to transfer and dilution in the infusion bag.
Following dilution of the suspension with normal saline or 5% dextrose, DORIBAX infusions stored at room temperature or under refrigeration should be completed according to the times in Table below.
 |
Constituted DORIBAX suspension or DORIBAX infusion should not be frozen. This storage information applies also to DORIBAX® diluted in Baxter Minibag Plus™.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022106s014lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.