Rifampin isoniazid pyrazinamide clinical studies: Difference between revisions
Gerald Chi (talk | contribs) m (Gerald Chi moved page Isoniazid clinical studies to Rifampin isoniazid pyrazinamide clinical studies without leaving a redirect) |
Gerald Chi (talk | contribs) mNo edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{ | {{Rifampin isoniazid pyrazinamide}} | ||
{{CMG}}; {{AE}} {{chetan}} | {{CMG}}; {{AE}} {{chetan}} | ||
==Clinical Trials== | ==Clinical Trials== | ||
A total of 250 patients were enrolled in an open label, prospective, randomized, parallel group, active controlled trial, for the treatment of pulmonary tuberculosis. There were 241 patients evaluable for efficacy, 123 patients received isoniazid, rifampin and pyrazinamide as separate tablets and capsules for 56 days, and 118 patients received 4 to 6 RIFATER tablets based on body weight for 56 days. RIFATER tablets and the drugs dosed as separate tablets and capsules were administered based on body weight during the intensive phase of treatment according to the following table.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = RIFATER (RIFAMPIN, ISONIAZID AND PYRAZINAMIDE) TABLET, SUGAR COATED [SANOFI-AVENTIS U.S. LLC] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=429a2f62-9fe6-4299-b314-92a9d22b1381 | publisher = | date = | accessdate }}</ref> | A total of 250 patients were enrolled in an open label, prospective, randomized, parallel group, active controlled trial, for the treatment of [[pulmonary tuberculosis]]. There were 241 patients evaluable for efficacy, 123 patients received isoniazid, rifampin and pyrazinamide as separate tablets and capsules for 56 days, and 118 patients received 4 to 6 RIFATER tablets based on body weight for 56 days. RIFATER tablets and the drugs dosed as separate tablets and capsules were administered based on body weight during the intensive phase of treatment according to the following table.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = RIFATER (RIFAMPIN, ISONIAZID AND PYRAZINAMIDE) TABLET, SUGAR COATED [SANOFI-AVENTIS U.S. LLC] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=429a2f62-9fe6-4299-b314-92a9d22b1381 | publisher = | date = | accessdate }}</ref> | ||
{| | {| | ||
|- | |- | ||
| [[File:|800px|thumb]] | | [[File:RIFATER001.jpg|800px|thumb]] | ||
|- | |- | ||
|} | |} | ||
| Line 19: | Line 19: | ||
{| | {| | ||
|- | |- | ||
| [[File:|800px|thumb]] | | [[File:RIFATER002.jpg|800px|thumb]] | ||
|- | |- | ||
|} | |} | ||
For adverse events, (See ADVERSE REACTIONS). | For adverse events, (See ADVERSE REACTIONS). | ||
Latest revision as of 23:47, 3 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Clinical Trials
A total of 250 patients were enrolled in an open label, prospective, randomized, parallel group, active controlled trial, for the treatment of pulmonary tuberculosis. There were 241 patients evaluable for efficacy, 123 patients received isoniazid, rifampin and pyrazinamide as separate tablets and capsules for 56 days, and 118 patients received 4 to 6 RIFATER tablets based on body weight for 56 days. RIFATER tablets and the drugs dosed as separate tablets and capsules were administered based on body weight during the intensive phase of treatment according to the following table.[1]
 |
During the continuation phase, both treatment groups received 450 mg of rifampin and 300 mg of isoniazid per day for 4 months if the patient weighed <50 kg or 600 mg of rifampin and 300 mg of isoniazid per day for 4 months if the patient weighed ≥50 kg. Patients were followed for occurrence of relapses for up to 30 months after the end of therapy.
There were no significant differences in the negative bacteriological sputum results (available in a subset of patients) between the two treatments at 2 and 6 months during the trial and during the follow-up period. See table below.
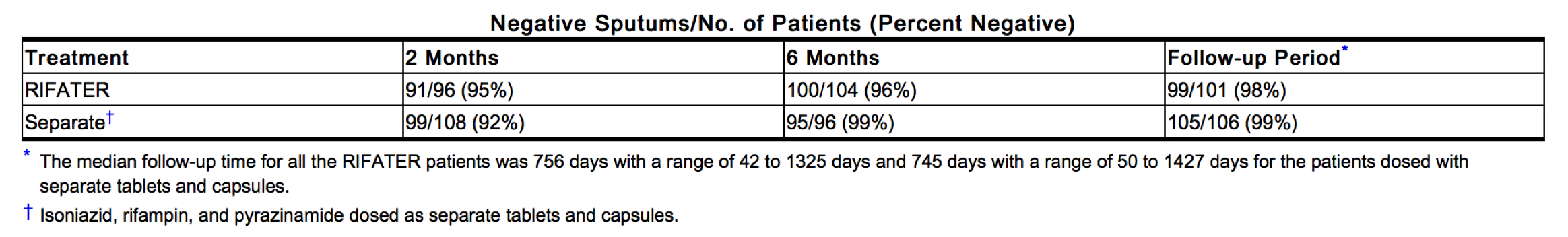 |
For adverse events, (See ADVERSE REACTIONS).
References
- ↑ "RIFATER (RIFAMPIN, ISONIAZID AND PYRAZINAMIDE) TABLET, SUGAR COATED [SANOFI-AVENTIS U.S. LLC]". Text " accessdate " ignored (help)
Adapted from the FDA Package Insert.