Darunavir adverse reactions: Difference between revisions
Ahmed Zaghw (talk | contribs) No edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 3: | Line 3: | ||
{{CMG}} {{AE}} {{AZ}} | {{CMG}} {{AE}} {{AZ}} | ||
==Adverse Reactions== | |||
The overall safety profile of PREZISTA/ritonavir 800/100 mg once daily and PREZISTA/ritonavir 600/100 mg twice daily is based on clinical trials and post-marketing data, and is consistent with the data presented below. | The overall safety profile of PREZISTA/ritonavir 800/100 mg once daily and PREZISTA/ritonavir 600/100 mg twice daily is based on clinical trials and post-marketing data, and is consistent with the data presented below. | ||
| Line 15: | Line 15: | ||
=====Treatment-Naïve Adults===== | =====Treatment-Naïve Adults===== | ||
====Study TMC114-C211==== | ======Study TMC114-C211====== | ||
The safety assessment is based on all safety data from the Phase 3 trial TMC114-C211 comparing PREZISTA/ritonavir 800/100 mg once daily versus lopinavir/ritonavir 800/200 mg per day in 689 antiretroviral treatment-naïve HIV-1-infected adult subjects. The total mean exposure for subjects in the PREZISTA/ritonavir 800/100 mg once daily arm and in the lopinavir/ritonavir 800/200 mg per day arm was 162.5 and 153.5 weeks, respectively. | The safety assessment is based on all safety data from the Phase 3 trial TMC114-C211 comparing PREZISTA/ritonavir 800/100 mg once daily versus lopinavir/ritonavir 800/200 mg per day in 689 antiretroviral treatment-naïve HIV-1-infected adult subjects. The total mean exposure for subjects in the PREZISTA/ritonavir 800/100 mg once daily arm and in the lopinavir/ritonavir 800/200 mg per day arm was 162.5 and 153.5 weeks, respectively. | ||
| Line 31: | Line 31: | ||
Treatment-emergent ADRs of at least moderate intensity (greater than or equal to Grade 2) occurring in less than 2% of antiretroviral treatment-naïve subjects receiving PREZISTA/ritonavir 800/100 mg once daily are listed below by body system: | Treatment-emergent ADRs of at least moderate intensity (greater than or equal to Grade 2) occurring in less than 2% of antiretroviral treatment-naïve subjects receiving PREZISTA/ritonavir 800/100 mg once daily are listed below by body system: | ||
Gastrointestinal Disorders: acute pancreatitis, dyspepsia, flatulence | Gastrointestinal Disorders: [[acute pancreatitis]], [[dyspepsia]], [[flatulence]] | ||
General Disorders and Administration Site Conditions: asthenia | General Disorders and Administration Site Conditions: [[asthenia]] | ||
Hepatobiliary Disorders: acute hepatitis (e.g., acute hepatitis, cytolytic hepatitis, hepatotoxicity) | Hepatobiliary Disorders: acute hepatitis (e.g., acute [[hepatitis]], cytolytic hepatitis, [[hepatotoxicity]]) | ||
Immune System Disorders: (drug) hypersensitivity, immune reconstitution syndrome | Immune System Disorders: (drug) [[hypersensitivity]], [[immune reconstitution syndrome]] | ||
Metabolism and Nutrition Disorders: diabetes mellitus | Metabolism and Nutrition Disorders: [[diabetes mellitus]] | ||
Musculoskeletal and Connective Tissue Disorders: myalgia, osteonecrosis | Musculoskeletal and Connective Tissue Disorders: [[myalgia]], [[osteonecrosis]] | ||
Psychiatric Disorders: abnormal dreams | Psychiatric Disorders: abnormal dreams | ||
Skin and Subcutaneous Tissue Disorders: angioedema, pruritus, Stevens-Johnson | Skin and Subcutaneous Tissue Disorders: [[angioedema]], [[pruritus]], [[Stevens-Johnson syndrome]], [[urticaria]] | ||
Laboratory abnormalities: | Laboratory abnormalities: | ||
Selected Grade 2 to 4 laboratory abnormalities that represent a worsening from baseline observed in antiretroviral treatment-naïve adult subjects treated with PREZISTA/ritonavir 800/100 mg once daily are presented in Table 8. | Selected Grade 2 to 4 laboratory abnormalities that represent a worsening from baseline observed in antiretroviral treatment-naïve adult subjects treated with PREZISTA/ritonavir 800/100 mg once daily are presented in Table 8. | ||
{| | {| | ||
Revision as of 23:57, 31 December 2013
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Adverse Reactions
The overall safety profile of PREZISTA/ritonavir 800/100 mg once daily and PREZISTA/ritonavir 600/100 mg twice daily is based on clinical trials and post-marketing data, and is consistent with the data presented below.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Due to the need for co-administration of PREZISTA with ritonavir, please refer to ritonavir prescribing information for ritonavir-associated adverse reactions.
Clinical Trials Experience
Treatment-Naïve Adults
Study TMC114-C211
The safety assessment is based on all safety data from the Phase 3 trial TMC114-C211 comparing PREZISTA/ritonavir 800/100 mg once daily versus lopinavir/ritonavir 800/200 mg per day in 689 antiretroviral treatment-naïve HIV-1-infected adult subjects. The total mean exposure for subjects in the PREZISTA/ritonavir 800/100 mg once daily arm and in the lopinavir/ritonavir 800/200 mg per day arm was 162.5 and 153.5 weeks, respectively.
The majority of the adverse drug reactions (ADRs) reported during treatment with PREZISTA/ritonavir 800/100 mg once daily were mild in severity. The most common clinical ADRs to PREZISTA/ritonavir 800/100 mg once daily (greater than or equal to 5%) of at least moderate intensity (greater than or equal to Grade 2) were diarrhea, headache, abdominal pain and rash. 2.3% of subjects in the PREZISTA/ritonavir arm discontinued treatment due to ADRs.
ADRs to PREZISTA/ritonavir 800/100 mg once daily of at least moderate intensity (greater than or equal to Grade 2) in antiretroviral treatment-naïve HIV-1-infected adult subjects are presented in Table 7 and subsequent text below the table.
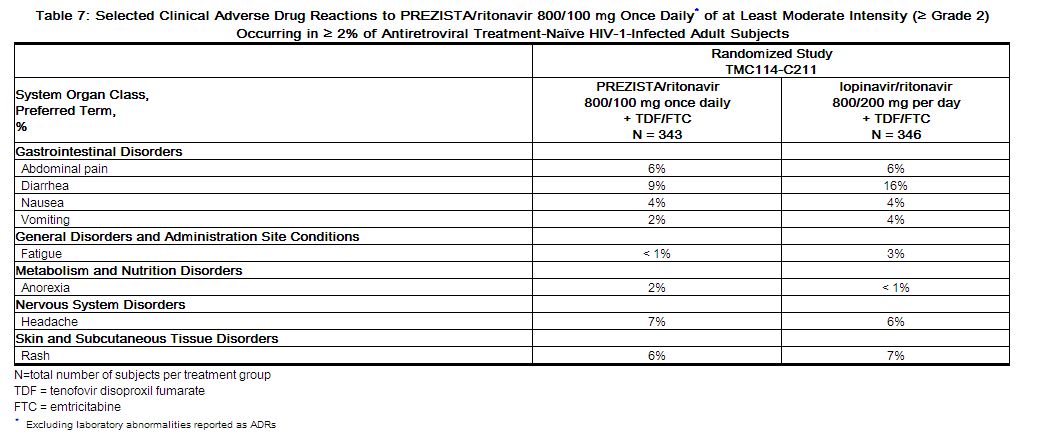 |
Less Common Adverse Reactions
Treatment-emergent ADRs of at least moderate intensity (greater than or equal to Grade 2) occurring in less than 2% of antiretroviral treatment-naïve subjects receiving PREZISTA/ritonavir 800/100 mg once daily are listed below by body system:
Gastrointestinal Disorders: acute pancreatitis, dyspepsia, flatulence
General Disorders and Administration Site Conditions: asthenia
Hepatobiliary Disorders: acute hepatitis (e.g., acute hepatitis, cytolytic hepatitis, hepatotoxicity)
Immune System Disorders: (drug) hypersensitivity, immune reconstitution syndrome
Metabolism and Nutrition Disorders: diabetes mellitus
Musculoskeletal and Connective Tissue Disorders: myalgia, osteonecrosis
Psychiatric Disorders: abnormal dreams
Skin and Subcutaneous Tissue Disorders: angioedema, pruritus, Stevens-Johnson syndrome, urticaria
Laboratory abnormalities:
Selected Grade 2 to 4 laboratory abnormalities that represent a worsening from baseline observed in antiretroviral treatment-naïve adult subjects treated with PREZISTA/ritonavir 800/100 mg once daily are presented in Table 8.
 |
 |
References
Adapted from the FDA Package Insert.