Pheochromocytoma pathophysiology: Difference between revisions
No edit summary |
Ifrah Fatima (talk | contribs) |
||
| (43 intermediate revisions by 4 users not shown) | |||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
It is understood that pheochromocytoma is mediated by excessive secretion of [[catecholamines]] and subsequent stimulation of [[adrenergic receptors]]. It arises from the [[chromaffin cells]] of the [[adrenal medulla]] and [[Sympathetic ganglion|sympathetic ganglia]]. The pathophysiology of pheochromocytoma does not depend on the histological subtype. [[Malignant]] and [[benign]] pheochromocytomas share the same [[biochemical]] and [[histological]] features. It may be sporadic or familial. All of these forms have [[genetic]] origin depending on a large number of [[genes]], for example, [[Von Hippel-Lindau tumor suppressor|VHL]], [[SDH|SDH,]] [[NF1]], [[RET proto-oncogene|RET]] [[Gene|genes]]. It is associated with conditions like MEN 2A syndrome, MEN 2B syndrome, VHL disease, and NF1. | |||
==Pathophysiology== | ==Pathophysiology== | ||
===Physiology=== | |||
Pheochromocytoma is not associated with normal physiology. | |||
== | ===Pathology=== | ||
* It is understood that pheochromocytoma is the is mediated by excessive secretion of [[catecholamines]] and subsequent stimulation of [[adrenergic receptors]]. | |||
* Commonly secreted [[catecholamines]] include [[norepinephrine]] (predominant) and [[epinephrine]]. Some [[tumors]] may also secrete [[dopamine]]. <ref name="pmidorcid.org/0000-0003-2771-564X">{{cite journal| author=Smith RJ, Bryant RG| title=Metal substitutions incarbonic anhydrase: a halide ion probe study. | journal=Biochem Biophys Res Commun | year= 1975 | volume= 66 | issue= 4 | pages= 1281-6 | pmid=orcid.org/0000-0003-2771-564X | doi=10.1016/0006-291x(75)90498-2 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3 }} </ref> | |||
* Excessive secretion of [[catecholamines]] may be either continuous or intermittent. | |||
*Pheochromocytoma is a [[tumor]] which arises from the [[chromaffin cells]] of the [[adrenal medulla]] and [[Sympathetic ganglion|sympathetic ganglia]]. | |||
*The pathophysiology of pheochromocytoma does not depend on the histological subtype. [[Malignant]] and [[benign]] pheochromocytomas share the same [[biochemical]] and [[histological]] features. <ref name="pmid10363888">{{cite journal| author=Goldstein RE, O'Neill JA, Holcomb GW, Morgan WM, Neblett WW, Oates JA et al.| title=Clinical experience over 48 years with pheochromocytoma. | journal=Ann Surg | year= 1999 | volume= 229 | issue= 6 | pages= 755-64; discussion 764-6 | pmid=10363888 | doi= | pmc=1420821 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10363888 }}</ref><ref name="pmid1900669">{{cite journal| author=Raz I, Katz A, Spencer MK| title=Epinephrine inhibits insulin-mediated glycogenesis but enhances glycolysis in human skeletal muscle. | journal=Am J Physiol | year= 1991 | volume= 260 | issue= 3 Pt 1 | pages= E430-5 | pmid=1900669 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1900669 }}</ref><ref name="pmid3521311">{{cite journal| author=Arnall DA, Marker JC, Conlee RK, Winder WW| title=Effect of infusing epinephrine on liver and muscle glycogenolysis during exercise in rats. | journal=Am J Physiol | year= 1986 | volume= 250 | issue= 6 Pt 1 | pages= E641-9 | pmid=3521311 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3521311 }}</ref> | |||
* The exact mechanism responsible for surge in [[catecholamine]] secretion remains unclear but it has been postulated that certain [[medications]] (such as [[opiates]], [[metoclopramide]] or [[beta blockers]]) and changes in [[tumor]] [[blood flow]] and [[pressure]] could be responsible factors. | |||
*Binding to [[Β1-adrenoreceptors|β<sub>1</sub>]] receptors causes [[renin]] release from [[juxtaglomerular cells]] and [[lipolysis]] in [[adipose tissue]]. It Increases [[cardiac output]] by: | |||
**Increase in [[heart rate]] in [[sinoatrial node]] | |||
**Increase in [[atrial]] cardiac muscle [[contractility]] | |||
**Increases in [[contractility]] and [[automaticity]] of [[ventricular]] cardiac muscle | |||
**Increases in [[Electrical conduction system of the heart|conduction]] and [[automaticity]] of [[atrioventricular node]] | |||
==Genetics== | ==Genetics== | ||
* 60-65 | * Pheochromocytoma can be transmitted in a sporadic(60-65%) or familial pattern. <ref name="pmid6103678">{{cite journal |vauthors=Webb TA, Sheps SG, Carney JA |title=Differences between sporadic pheochromocytoma and pheochromocytoma in multiple endocrime neoplasia, type 2 |journal=Am. J. Surg. Pathol. |volume=4 |issue=2 |pages=121–6 |year=1980 |pmid=6103678 |doi= |url=}}</ref><ref name="pmid3474647">{{cite journal |vauthors=Yee JK, Moores JC, Jolly DJ, Wolff JA, Respess JG, Friedmann T |title=Gene expression from transcriptionally disabled retroviral vectors |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=84 |issue=15 |pages=5197–201 |year=1987 |pmid=3474647 |pmc=298821 |doi= |url=}}</ref> | ||
* | * Genes involved in the pathogenesis of pheochromocytoma include: | ||
* | **[[RET gene|RET]] gene ([[MEN, type 2a|MEN 2A]], [[Multiple endocrine neoplasia type 2|MEN 2B]] [[Syndrome|syndromes]]) | ||
* | **[[NF1|NF1 gene]] | ||
**[[Von Hippel-Lindau tumor suppressor|VHL gene]] ([[Von Hippel-Lindau disease|VHL disease]]) | |||
**[[SDHD]], [[SDHB]], and [[SDHC]] genes of the [[Mitochondrial|mitochondrial complex]] <ref name="pmid15883706">{{cite journal| author=Gimm O| title=Pheochromocytoma-associated syndromes: genes, proteins and functions of RET, VHL and SDHx. | journal=Fam Cancer | year= 2005 | volume= 4 | issue= 1 | pages= 17-23 | pmid=15883706 | doi=10.1007/s10689-004-5740-1 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15883706 }} </ref> | |||
**[[SDHA]], [[SDHAF2]], [[TMEM127]] (transmembrane protein 127), [[MAX (gene)|MAX]] (myc-associated factor X), [[Fumarate hydratase|FH]] (fumarate hydratase), [[PDH complex|PDH1]], PDH2 (pyruvate dehydrogenase), [[Hypoxia inducible factors|HIF1alpha]] (hypoxia-inducible factor), [[MDH1|MDH2]] (malate dehydrogenase), and KIF1Bß (kinesin family member) genes. <ref>{{cite book | last = Jameson | first = J | title = Harrison's Principles of Internal Medicine 19th Edition and Harrison's Manual of Medicine 19th Edition VAL PAK | publisher = McGraw-Hill Medical | location = New York | year = 2017 | isbn = 978-1260128857 }} </ref> | |||
* [[ | |||
[[ | |||
[[Pheochromocytoma]] and [[Paraganglioma|paragangliomas]] (PPGL) susceptibility genes can be classified into the following clusters- <ref>{{cite book | last = Jameson | first = J | title = Harrison's Principles of Internal Medicine 19th Edition and Harrison's Manual of Medicine 19th Edition VAL PAK | publisher = McGraw-Hill Medical | location = New York | year = 2017 | isbn = 978-1260128857 }} </ref> <ref name="pmid15613462">{{cite journal| author=Eisenhofer G, Huynh TT, Pacak K, Brouwers FM, Walther MM, Linehan WM | display-authors=etal| title=Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. | journal=Endocr Relat Cancer | year= 2004 | volume= 11 | issue= 4 | pages= 897-911 | pmid=15613462 | doi=10.1677/erc.1.00838 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15613462 }} </ref> <ref name="pmid28477311">{{cite journal| author=Lam AK| title=Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. | journal=Endocr Pathol | year= 2017 | volume= 28 | issue= 3 | pages= 213-227 | pmid=28477311 | doi=10.1007/s12022-017-9484-5 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28477311 }} </ref> | |||
* Cluster 1 | |||
**[[Mutation|Mutations]] involving in [[overexpression]] of [[Vascular endothelial growth factor (VEGF) IRES A|vascular endothelial growth factor (VEGF)]] as a result of pseudohypoxia | |||
** Impaired [[DNA]] [[methylation]] leading to increased vascularization | |||
* Cluster 2 | |||
** Activating [[Mutation|mutations]] of [[Wnt signaling pathway|Wnt-signaling pathway]] including Wnt receptor signaling and [[Hedgehog signaling pathway|Hedgehog]] signaling. | |||
** Mutations of [[CSDE1]] (Cold shock domain containing E1) and [[MAML2|MAML3]] (Mastermind like transcriptional coactivator 3) genes7. | |||
* Cluster 3 | |||
** | ** Abnormal activation of [[Kinase|kinase signaling pathways]] like PI3Kinase/[[AKT]], [[RAS]]/RAF/ERK, and [[mTOR]] pathways. | ||
==Associated conditions== | ==Associated conditions== | ||
* | Conditions associated with pheochromocytoma include: | ||
*[[Multiple endocrine neoplasia]] ([[Multiple endocrine neoplasia type 1|MEN1]]) | |||
*[[Multiple endocrine neoplasia (MEN 2b)|Multiple endocrine neoplasia]] ([[MEN2|MEN2B]]) | |||
*[[Von Hippel-Lindau disease|Von-Hippel Lindau disease]] (VHL) | |||
*[[Neurofibromatosis type I|Neurofibromatosis 1]] (NF1) | |||
{| class="wikitable" | {| class="wikitable" | ||
! | ! align="center" style="background:#4479BA; color: #FFFFFF;" + |MEN 1 | ||
|''' | | align="center" style="background:#4479BA; color: #FFFFFF;" + |'''MEN 2''' | ||
|- | |- | ||
| rowspan="3" | | | rowspan="3" | | ||
| Line 86: | Line 69: | ||
==Gross Pathology== | ==Gross Pathology== | ||
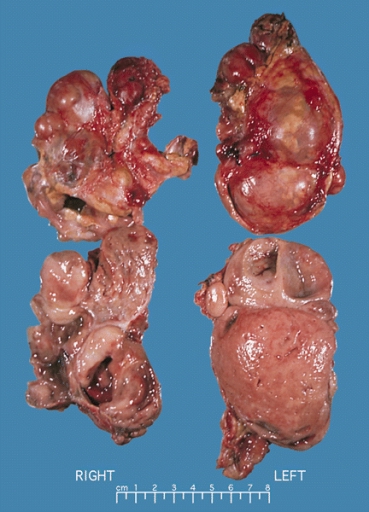
On gross pathology, | On [[gross pathology]], the characteristic findings of pheochromocytoma are: | ||
* Small to large tumors usually associated with [[hemorrhage]] and [[necrosis]].<ref name="pmid26266130">{{cite journal| author=Sajjanar AB, Athanikar VS, Dinesh US, Nanjappa B, Patil PB| title=Non Functional Unilateral Adrenal Myelolipoma, A Case Report. | journal=J Clin Diagn Res | year= 2015 | volume= 9 | issue= 6 | pages= ED03-4 | pmid=26266130 | doi=10.7860/JCDR/2015/13209.6070 | pmc=4525519 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26266130 }}</ref> | |||
* Usually [[Lobule|lobulated]] | |||
*[[Bilateral]] when [[familial]] [[Tumor|tumors]] | |||
* Associated with [[hyperplasia]] in the adjacent [[medulla]]. | |||
*[[Chromaffin]] reaction: fresh [[tumor]] cut section turns dark brown if add [[potassium dichromate]] at pH 5-6. | |||
<gallery> | <gallery> | ||
Image:Bilateral pheo MEN2.jpg|Bilateral pheochromocytoma in [[Multiple_endocrine_neoplasia_type_2|MEN2]]. Gross image. | Image:Bilateral pheo MEN2.jpg|Bilateral pheochromocytoma in [[Multiple_endocrine_neoplasia_type_2|MEN2]]. Gross image. Source: https://upload.wikimedia.org/wikipedia/commons/5/5f/Bilateral_pheo_MEN2.jpg | ||
</gallery> | </gallery> | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
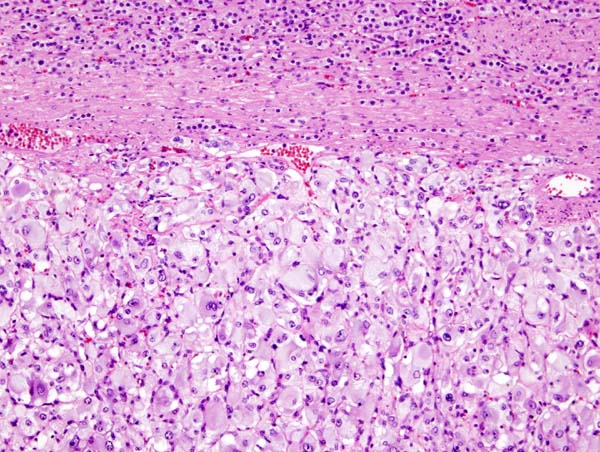
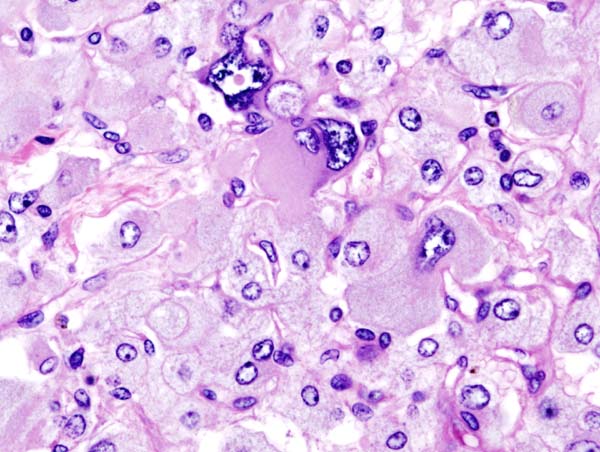
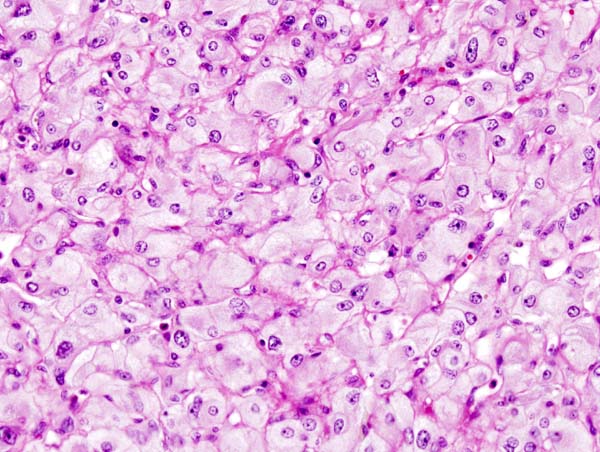
On microscopic | On microscopic histopathological analysis, the characterisitc findings of pheochromocytoma typically include: <ref name="pmid2912871">{{cite journal| author=Kliewer KE, Wen DR, Cancilla PA, Cochran AJ| title=Paragangliomas: assessment of prognosis by histologic, immunohistochemical, and ultrastructural techniques. | journal=Hum Pathol | year= 1989 | volume= 20 | issue= 1 | pages= 29-39 | pmid=2912871 | doi=10.1016/0046-8177(89)90199-8 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2912871 }} </ref> <ref name="pmid2684087">{{cite journal| author=Kliewer KE, Cochran AJ| title=A review of the histology, ultrastructure, immunohistology, and molecular biology of extra-adrenal paragangliomas. | journal=Arch Pathol Lab Med | year= 1989 | volume= 113 | issue= 11 | pages= 1209-18 | pmid=2684087 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2684087 }} </ref> | ||
* A nesting (Zellballen) pattern- this pattern is composed of well-defined clusters of tumor cells (round or polygonal epithelioid cells) containing eosinophilic cytoplasm separated by fibrovascular stroma. | |||
* These cells have a central nucleus with an eosinophilic, granular cytoplasm, and clumped chromatin. | |||
* At the periphery, spindle-shaped sustentacular or supporting cells are seen. | |||
<gallery> | <gallery> | ||
Image:Adrenal pheochromocytoma (1) histopathology.jpg|[[Micrograph]] of pheochromocytoma. | Image:Adrenal pheochromocytoma (1) histopathology.jpg|[[Micrograph]] of pheochromocytoma. Source: By Nephron - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=5938524 | ||
Image:Adrenal pheochromocytoma (3) histopathology.jpg|Histopathology of adrenal pheochromocytoma. Adrenectomy specimen. | Image:Adrenal pheochromocytoma (3) histopathology.jpg|Histopathology of adrenal pheochromocytoma. Adrenectomy specimen. Source: CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=535945 | ||
Image:Adrenal pheochromocytoma (2) histopathology.jpg|Micrograph of pheochromocytoma. | Image:Adrenal pheochromocytoma (2) histopathology.jpg|Micrograph of pheochromocytoma. Source: CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=535944 | ||
</gallery> | </gallery> | ||
Latest revision as of 23:28, 24 July 2020
|
Pheochromocytoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Pheochromocytoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Pheochromocytoma pathophysiology |
|
Risk calculators and risk factors for Pheochromocytoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmad Al Maradni, M.D. [2] Mohammed Abdelwahed M.D[3]
Overview
It is understood that pheochromocytoma is mediated by excessive secretion of catecholamines and subsequent stimulation of adrenergic receptors. It arises from the chromaffin cells of the adrenal medulla and sympathetic ganglia. The pathophysiology of pheochromocytoma does not depend on the histological subtype. Malignant and benign pheochromocytomas share the same biochemical and histological features. It may be sporadic or familial. All of these forms have genetic origin depending on a large number of genes, for example, VHL, SDH, NF1, RET genes. It is associated with conditions like MEN 2A syndrome, MEN 2B syndrome, VHL disease, and NF1.
Pathophysiology
Physiology
Pheochromocytoma is not associated with normal physiology.
Pathology
- It is understood that pheochromocytoma is the is mediated by excessive secretion of catecholamines and subsequent stimulation of adrenergic receptors.
- Commonly secreted catecholamines include norepinephrine (predominant) and epinephrine. Some tumors may also secrete dopamine. [1]
- Excessive secretion of catecholamines may be either continuous or intermittent.
- Pheochromocytoma is a tumor which arises from the chromaffin cells of the adrenal medulla and sympathetic ganglia.
- The pathophysiology of pheochromocytoma does not depend on the histological subtype. Malignant and benign pheochromocytomas share the same biochemical and histological features. [2][3][4]
- The exact mechanism responsible for surge in catecholamine secretion remains unclear but it has been postulated that certain medications (such as opiates, metoclopramide or beta blockers) and changes in tumor blood flow and pressure could be responsible factors.
- Binding to β1 receptors causes renin release from juxtaglomerular cells and lipolysis in adipose tissue. It Increases cardiac output by:
- Increase in heart rate in sinoatrial node
- Increase in atrial cardiac muscle contractility
- Increases in contractility and automaticity of ventricular cardiac muscle
- Increases in conduction and automaticity of atrioventricular node
Genetics
- Genes involved in the pathogenesis of pheochromocytoma include:
- RET gene (MEN 2A, MEN 2B syndromes)
- NF1 gene
- VHL gene (VHL disease)
- SDHD, SDHB, and SDHC genes of the mitochondrial complex [7]
- SDHA, SDHAF2, TMEM127 (transmembrane protein 127), MAX (myc-associated factor X), FH (fumarate hydratase), PDH1, PDH2 (pyruvate dehydrogenase), HIF1alpha (hypoxia-inducible factor), MDH2 (malate dehydrogenase), and KIF1Bß (kinesin family member) genes. [8]
Pheochromocytoma and paragangliomas (PPGL) susceptibility genes can be classified into the following clusters- [9] [10] [11]
- Cluster 1
- Mutations involving in overexpression of vascular endothelial growth factor (VEGF) as a result of pseudohypoxia
- Impaired DNA methylation leading to increased vascularization
- Cluster 2
- Activating mutations of Wnt-signaling pathway including Wnt receptor signaling and Hedgehog signaling.
- Mutations of CSDE1 (Cold shock domain containing E1) and MAML3 (Mastermind like transcriptional coactivator 3) genes7.
- Cluster 3
- Abnormal activation of kinase signaling pathways like PI3Kinase/AKT, RAS/RAF/ERK, and mTOR pathways.
Associated conditions
Conditions associated with pheochromocytoma include:
- Multiple endocrine neoplasia (MEN1)
- Multiple endocrine neoplasia (MEN2B)
- Von-Hippel Lindau disease (VHL)
- Neurofibromatosis 1 (NF1)
| MEN 1 | MEN 2 |
|---|---|
Gross Pathology
On gross pathology, the characteristic findings of pheochromocytoma are:
- Small to large tumors usually associated with hemorrhage and necrosis.[12]
- Usually lobulated
- Bilateral when familial tumors
- Associated with hyperplasia in the adjacent medulla.
- Chromaffin reaction: fresh tumor cut section turns dark brown if add potassium dichromate at pH 5-6.
-
Bilateral pheochromocytoma in MEN2. Gross image. Source: https://upload.wikimedia.org/wikipedia/commons/5/5f/Bilateral_pheo_MEN2.jpg
Microscopic Pathology
On microscopic histopathological analysis, the characterisitc findings of pheochromocytoma typically include: [13] [14]
- A nesting (Zellballen) pattern- this pattern is composed of well-defined clusters of tumor cells (round or polygonal epithelioid cells) containing eosinophilic cytoplasm separated by fibrovascular stroma.
- These cells have a central nucleus with an eosinophilic, granular cytoplasm, and clumped chromatin.
- At the periphery, spindle-shaped sustentacular or supporting cells are seen.
-
Micrograph of pheochromocytoma. Source: By Nephron - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=5938524
-
Histopathology of adrenal pheochromocytoma. Adrenectomy specimen. Source: CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=535945
-
Micrograph of pheochromocytoma. Source: CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=535944
Videos
{{#ev:youtube|7yjxG3KmX98}}
References
- ↑ Smith RJ, Bryant RG (1975). "Metal substitutions incarbonic anhydrase: a halide ion probe study". Biochem Biophys Res Commun. 66 (4): 1281–6. doi:10.1016/0006-291x(75)90498-2. PMID orcid.org/0000-0003-2771-564X Check
|pmid=value (help). - ↑ Goldstein RE, O'Neill JA, Holcomb GW, Morgan WM, Neblett WW, Oates JA; et al. (1999). "Clinical experience over 48 years with pheochromocytoma". Ann Surg. 229 (6): 755–64, discussion 764-6. PMC 1420821. PMID 10363888.
- ↑ Raz I, Katz A, Spencer MK (1991). "Epinephrine inhibits insulin-mediated glycogenesis but enhances glycolysis in human skeletal muscle". Am J Physiol. 260 (3 Pt 1): E430–5. PMID 1900669.
- ↑ Arnall DA, Marker JC, Conlee RK, Winder WW (1986). "Effect of infusing epinephrine on liver and muscle glycogenolysis during exercise in rats". Am J Physiol. 250 (6 Pt 1): E641–9. PMID 3521311.
- ↑ Webb TA, Sheps SG, Carney JA (1980). "Differences between sporadic pheochromocytoma and pheochromocytoma in multiple endocrime neoplasia, type 2". Am. J. Surg. Pathol. 4 (2): 121–6. PMID 6103678.
- ↑ Yee JK, Moores JC, Jolly DJ, Wolff JA, Respess JG, Friedmann T (1987). "Gene expression from transcriptionally disabled retroviral vectors". Proc. Natl. Acad. Sci. U.S.A. 84 (15): 5197–201. PMC 298821. PMID 3474647.
- ↑ Gimm O (2005). "Pheochromocytoma-associated syndromes: genes, proteins and functions of RET, VHL and SDHx". Fam Cancer. 4 (1): 17–23. doi:10.1007/s10689-004-5740-1. PMID 15883706.
- ↑ Jameson, J (2017). Harrison's Principles of Internal Medicine 19th Edition and Harrison's Manual of Medicine 19th Edition VAL PAK. New York: McGraw-Hill Medical. ISBN 978-1260128857.
- ↑ Jameson, J (2017). Harrison's Principles of Internal Medicine 19th Edition and Harrison's Manual of Medicine 19th Edition VAL PAK. New York: McGraw-Hill Medical. ISBN 978-1260128857.
- ↑ Eisenhofer G, Huynh TT, Pacak K, Brouwers FM, Walther MM, Linehan WM; et al. (2004). "Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome". Endocr Relat Cancer. 11 (4): 897–911. doi:10.1677/erc.1.00838. PMID 15613462.
- ↑ Lam AK (2017). "Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours". Endocr Pathol. 28 (3): 213–227. doi:10.1007/s12022-017-9484-5. PMID 28477311.
- ↑ Sajjanar AB, Athanikar VS, Dinesh US, Nanjappa B, Patil PB (2015). "Non Functional Unilateral Adrenal Myelolipoma, A Case Report". J Clin Diagn Res. 9 (6): ED03–4. doi:10.7860/JCDR/2015/13209.6070. PMC 4525519. PMID 26266130.
- ↑ Kliewer KE, Wen DR, Cancilla PA, Cochran AJ (1989). "Paragangliomas: assessment of prognosis by histologic, immunohistochemical, and ultrastructural techniques". Hum Pathol. 20 (1): 29–39. doi:10.1016/0046-8177(89)90199-8. PMID 2912871.
- ↑ Kliewer KE, Cochran AJ (1989). "A review of the histology, ultrastructure, immunohistology, and molecular biology of extra-adrenal paragangliomas". Arch Pathol Lab Med. 113 (11): 1209–18. PMID 2684087.