Traveller vaccination hepatitis A: Difference between revisions
(Created page with "__NOTOC__ {{Traveller vaccination human papillomavirus}} {{CMG}};{{AE}}{{MehdiP}} ==Overview== ==Disease cause== ==Transmission== ==Nature of the disease== ==Geographical...") |
No edit summary |
||
| (9 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
'''To read more about hepatitis A, click [[Hepatitis A|here]].''' | |||
__NOTOC__ | __NOTOC__ | ||
{{Traveller vaccination | {{Traveller vaccination}} | ||
{{CMG}};{{AE}}{{ | {{CMG}};{{AE}}{{USAMA}} | ||
==Disease cause== | ==Disease cause== | ||
[[Hepatitis A virus]] (HAV). | |||
==Transmission== | ==Transmission== | ||
The virus is acquired through close contact with infected individuals or through faecally contaminated food or drinkingwater. There is no insect vector or animal reservoir. | |||
==Nature of the disease== | ==Nature of the disease== | ||
Acute viral hepatitis is characterized by abrupt onset of [[fever]], [[malaise]], [[nausea]] and [[abdominal discomfort]], followed by [[jaundice]] a few days later. In very young children infection is usually mild or asymptomatic whereas in older children symptomatic disease is common. The disease is often more severe in adults and full recovery may take several months. The case-fatality rate is greater than 2% for those over 40 years of age and about 4% for those aged 60 years or more. | |||
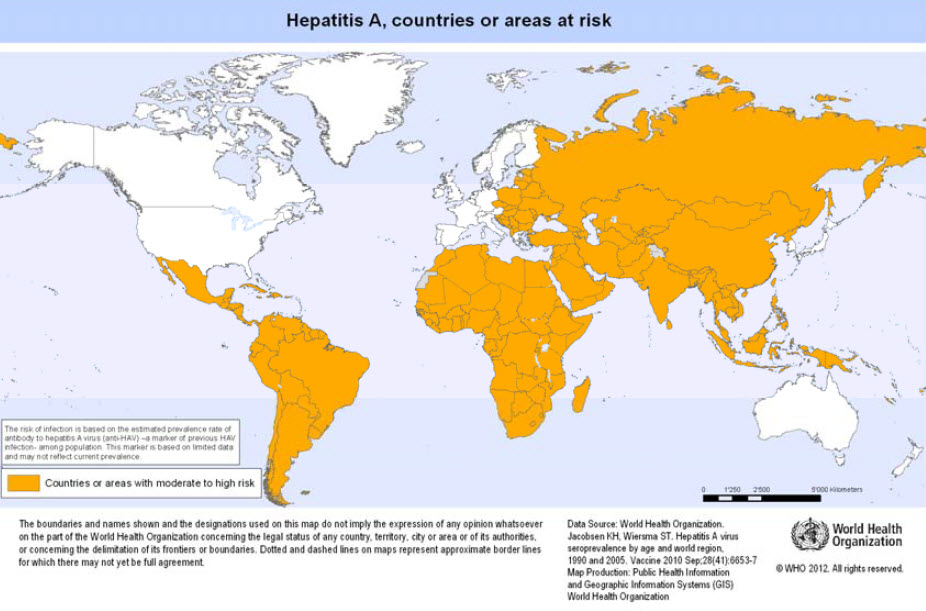
==Geographical distribution== | ==Geographical distribution== | ||
Worldwide, but most common in areas where sanitary conditions are poor. | |||
[[image:Hepatitis A.jpg]] | |||
<br clear="left"/> | |||
==Risk for travellers== | ==Risk for travellers== | ||
Non-immune travellers to developing countries are at significant risk of infection, particularly in settings with poor food and drinking-water control and poor sanitation People born and raised in developing countries, and those born before 1945 in industrialized countries, have usually been infected with [[HAV]] in childhood and are likely to be immune. | |||
== Precautions == | |||
Avoid or boil potentially contaminated food and water. Short-term protection through injection of human immune globulin is gradually being replaced by hepatitis A vaccination. | |||
==Vaccine== | ==Vaccine== | ||
Two types of hepatitis A vaccines are currently used worldwide, namely formaldehyde-inactivated vaccines and live attenuated vaccines. Both types are safe and highly immunogenic and provide long-lasting, possibly life-long, protection against hepatitis A in both children and adults. | |||
* '''Formaldehyde-inactivated vaccines''': Inactivated hepatitis A virus vaccines are used in most countries. Monovalent inactivated vaccines are available in both paediatric dose (0.5 ml) for children aged 1 year to 15 years and adult dose (1 ml). Traditionally, a two-dose schedule is recommended, particularly for immunocompromised individuals. However, in healthy individuals, comparable effectiveness has been achieved with a single dose. A combined hepatitis A/typhoid (ViCPS) vaccine, administered as a single dose, confers high levels of protection against both these waterborne diseases. A combination vaccine that provides protection against both hepatitis A and hepatitis B should be considered for travellers who may be exposed to both organisms. | |||
* '''Live attenuated vaccines (based on the H2 or LA-1 strain of HAV)''': These vaccines are manufactured in China and available in several other countries. Presence of anti-HAV (IgG) antibodies was documented after 15 years in 72% to 88% of the vaccinees, implying that, in most cases, long-term protection against [[hepatitis A]] is achieved with live attenuated vaccines. | |||
==Summary of vaccine data== | ==Summary of vaccine data== | ||
{| class="wikitable" | |||
! | |||
!Considerations | |||
|- | |||
| rowspan="2" |Type of vaccine | |||
| | |||
* '''Inactivated''' hepatitis A virus vaccines are licensed for intramuscular administration in a two-dose series. | |||
|- | |||
| | |||
* The '''live attenuated''' vaccine is administered as a single subcutaneous dose. | |||
|- | |||
|Number of doses | |||
| | |||
* Minimum age is 1 year for both inactivated and live attenuated hepatitis A virus vaccines. Inactivated vaccine: a complete vaccination schedule as recommended by the manufacturer consists of 2 doses. The interval between the first (primary) dose and the second (booster) dose is flexible (from 6 months up to 4–5 years), but is usually 6–18 months. In healthy individuals, a single dose seems to be similarly efficacious and only one dose is recommended for long-term protection. | |||
* Live vaccine: one dose. | |||
|- | |||
|Boosters | |||
| | |||
* Not necessary. | |||
|- | |||
|Contraindications | |||
| | |||
* Severe allergic reaction to previous dose. | |||
|- | |||
|Adverse reactions | |||
| | |||
* '''Inactivated vaccine''': mild local reaction of short duration, mild systemic reaction. | |||
* '''Live vaccine''': few reported. | |||
|- | |||
|Before departure | |||
| | |||
* Inactivated and live vaccines: protection is achieved within 2–4 weeks after first dose. Given the long incubation period of hepatitis A (average 2–4 weeks), the vaccine can be administered up to the day of departure and still protect travellers. | |||
|- | |||
|Indication | |||
| | |||
* Hepatitis A vaccination should be considered for individuals aged ≥1 year who are travelling to countries or areas of intermediate or high endemicity. Those at high risk of acquiring severe disease, such as immunosuppressed patients and patients with chronic liver disease, should be strongly encouraged to be vaccinated regardless of where they travel. In addition, people at increased risk of hepatitis A including men who have sex with men, those requiring life-long treatment with blood products, and people who inject drugs should be vaccinated. | |||
|- | |||
|Special precautions | |||
|None. | |||
|} | |||
Latest revision as of 15:42, 24 April 2017
To read more about hepatitis A, click here.
|
Traveler Vaccination |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Usama Talib, BSc, MD [2]
Disease cause
Hepatitis A virus (HAV).
Transmission
The virus is acquired through close contact with infected individuals or through faecally contaminated food or drinkingwater. There is no insect vector or animal reservoir.
Nature of the disease
Acute viral hepatitis is characterized by abrupt onset of fever, malaise, nausea and abdominal discomfort, followed by jaundice a few days later. In very young children infection is usually mild or asymptomatic whereas in older children symptomatic disease is common. The disease is often more severe in adults and full recovery may take several months. The case-fatality rate is greater than 2% for those over 40 years of age and about 4% for those aged 60 years or more.
Geographical distribution
Worldwide, but most common in areas where sanitary conditions are poor.
Risk for travellers
Non-immune travellers to developing countries are at significant risk of infection, particularly in settings with poor food and drinking-water control and poor sanitation People born and raised in developing countries, and those born before 1945 in industrialized countries, have usually been infected with HAV in childhood and are likely to be immune.
Precautions
Avoid or boil potentially contaminated food and water. Short-term protection through injection of human immune globulin is gradually being replaced by hepatitis A vaccination.
Vaccine
Two types of hepatitis A vaccines are currently used worldwide, namely formaldehyde-inactivated vaccines and live attenuated vaccines. Both types are safe and highly immunogenic and provide long-lasting, possibly life-long, protection against hepatitis A in both children and adults.
- Formaldehyde-inactivated vaccines: Inactivated hepatitis A virus vaccines are used in most countries. Monovalent inactivated vaccines are available in both paediatric dose (0.5 ml) for children aged 1 year to 15 years and adult dose (1 ml). Traditionally, a two-dose schedule is recommended, particularly for immunocompromised individuals. However, in healthy individuals, comparable effectiveness has been achieved with a single dose. A combined hepatitis A/typhoid (ViCPS) vaccine, administered as a single dose, confers high levels of protection against both these waterborne diseases. A combination vaccine that provides protection against both hepatitis A and hepatitis B should be considered for travellers who may be exposed to both organisms.
- Live attenuated vaccines (based on the H2 or LA-1 strain of HAV): These vaccines are manufactured in China and available in several other countries. Presence of anti-HAV (IgG) antibodies was documented after 15 years in 72% to 88% of the vaccinees, implying that, in most cases, long-term protection against hepatitis A is achieved with live attenuated vaccines.
Summary of vaccine data
| Considerations | |
|---|---|
| Type of vaccine |
|
| |
| Number of doses |
|
| Boosters |
|
| Contraindications |
|
| Adverse reactions |
|
| Before departure |
|
| Indication |
|
| Special precautions | None. |