Traveller vaccination cholera
Jump to navigation
Jump to search
To read more about cholera, click here.
|
Traveler Vaccination |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Seyedmahdi Pahlavani, M.D. [2]
Disease cause
Vibrio cholerae bacteria of serogroups O1 and O139.
Transmission
- Directly or indirectly contaminated food or water with faeces or vomitus.
- Cholera affects only human beings; there is no insect vector or animal reservoir host.
Nature of the disease
Mostly asymptomatic. Mild cases present with watery diarrhea. In severe cases, there is sudden onset of profuse watery diarrhea with nausea and vomiting and rapid development of dehydration. In severe untreated cases, death may occur within a few hours due to dehydration leading to circulatory collapse.
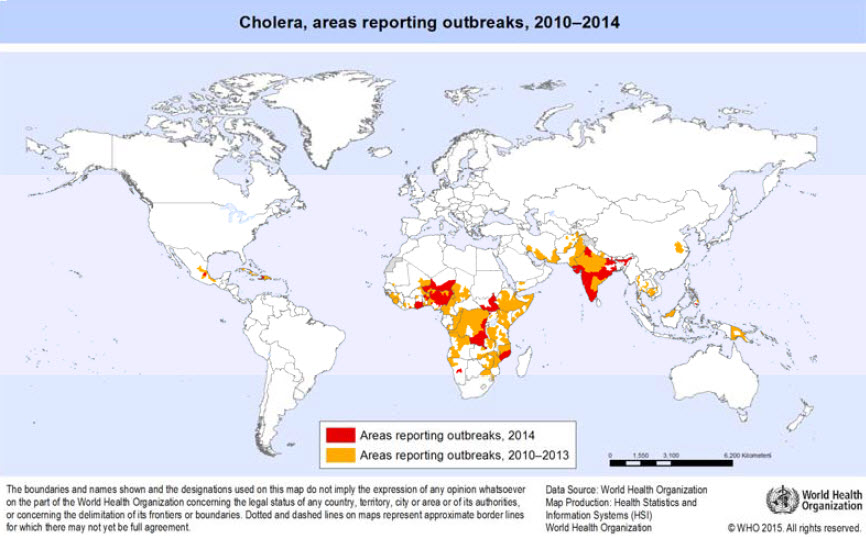
Geographical distribution
- Mainly in low-income countries with poor access to sanitary and clean water.
- Many developing countries are affected, particularly in Africa and Asia and, to a lesser extent, in Central and South America.
Risk for travellers
Risk for travellers is low even in epidemic regions. However, humanitarian relief workers in disaster areas and refugee camps may be at risk.
General precautions
- Cholera vaccination is not required as a condition of entry to any country.
- Avoid drinking or eating unsafe water or foods.
Vaccine
- Oral vaccine consisting of killed whole-cell V. cholerae O1 in combination with a recombinant B-subunit of cholera toxin (WC/rBS).
- Primary immunization consists of two oral doses ≥7 days (but <6 weeks) apart for adults and children aged 6 years and over.
- For children aged 2–5 years, three doses are recommended.
- Following primary immunization, protection against cholera may be expected after about 1 week.
- The vaccine is not licensed for children under 2 years of age.
Summary of vaccine data
| Considerations | |
|---|---|
| Type of vaccine | Killed oral O1 whole-cell with Bsubunit. |
| Killed oral O1 and O139. | |
| Number of doses |
|
| |
| Contraindications | Hypersensitivity to previous dose. |
| Adverse reactions | Mild gastrointestinal disturbances. |
| Before departure | 2 weeks. |
| Indication | Travellers at high risk (e.g. emergency/relief workers). |