Gestational trophoblastic disease
|
Gestational trophoblastic disease |
For patient information on Hydatiform mole, click here
For patient information on Choriocarcinoma, click here
| Gestational trophoblastic disease | |
| Classification and external resources | |
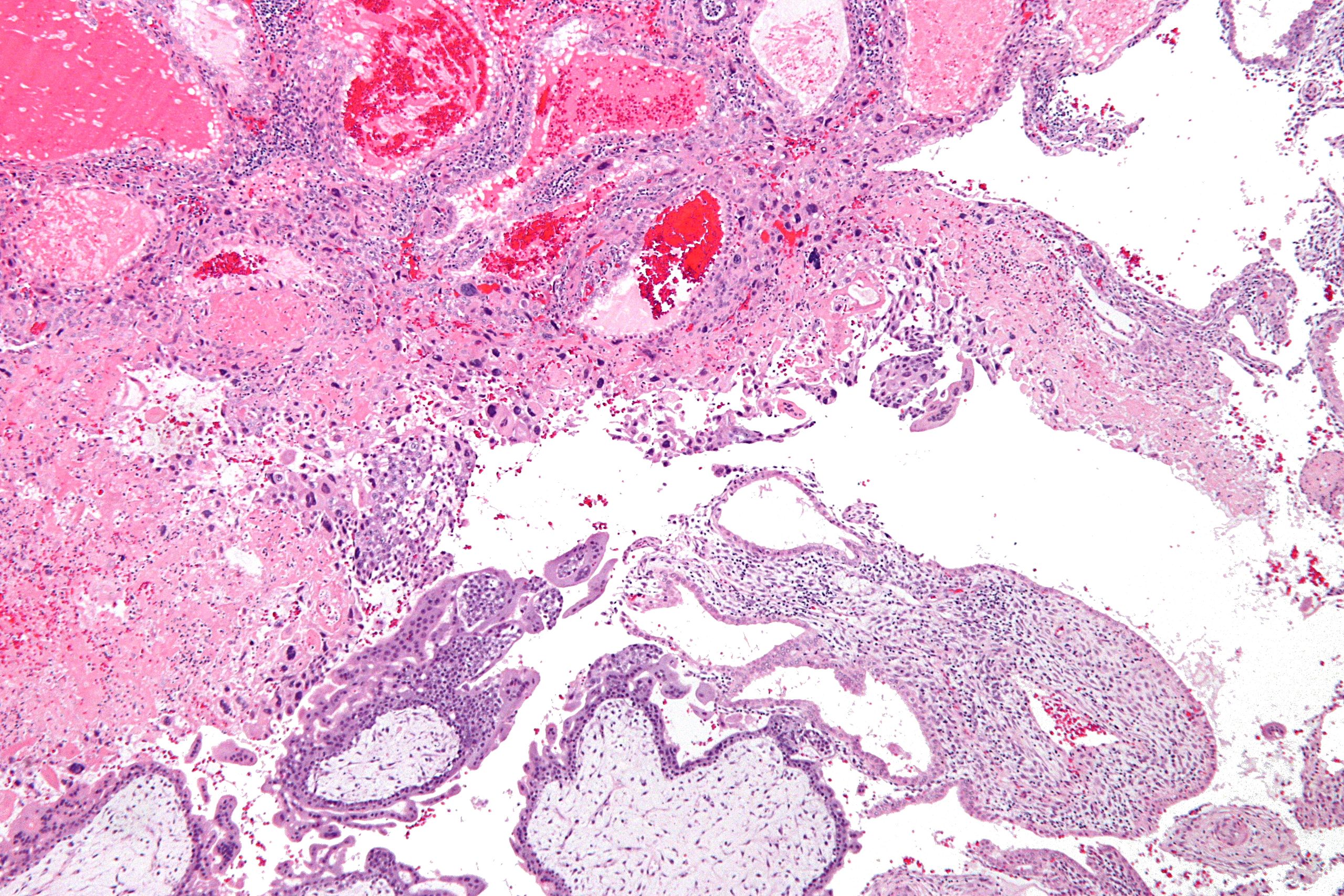
| |
|---|---|
| Micrograph of intermediate trophoblast, decidua and a hydatidiform mole (bottom of image). H&E stain. |
Synonyms and keywords: GTD, pregnancy-related tumors, trophoblastic diseases
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [2]
Overview
Gestational trophoblastic disease (GTD) includes a spectrum of disease states affecting pregnant individuals and range from benign conditions such as complete and partial molar pregnancy, exaggerated placental site tumor (EPS), and placental-site nodule (PSN) to malignant disorders (gestational trophoblastic neoplasia) such as invasive mole, choriocarcinoma (CC), placenta-site trophoblastic tumor (PSTT), and epitheloid trophoblastic tumors (ETT). Hydatidiform moles are a benign entities which may be either complete or partial. These benign moles may occasionally transform into invasive moles, which are malignant and may even progress to choriocarcinoma. Gestational trophoblasts may invade uterine tissue. GTD may simulate pregnancy due to increased uterine size, however they contain abnormal fetal tissue and typically the uterus is inappropriately large for date. This tissue may grow at the same rate as a normal pregnancy, and produces beta human chorionic gonadotropin (beta-hCG), a hormone produced by the placenta, which is measured to monitor fetal well-being after implantation. While GTD overwhelmingly affects women of child-bearing age, it may rarely occur in postmenopausal women.
Classification
Gestational trophoblastic disease (GTD) may be classified as follows:
- Hydatidiform mole (HM).
- Invasive mole
- Choriocarcinoma
- Placental-site trophoblastic tumor (PSTT); (very rare)
- Epithelioid trophoblastic tumor (ETT); (extremely rare)
Epidemiology
The reported incidence of GTD varies widely worldwide, from a low of 23 per 100,000 pregnancies (Paraguay) to a high of 1,299 per 100,000 pregnancies (Indonesia). However, at least part of this variability is caused by differences in diagnostic criteria and reporting. The reported incidence in the United States is about 110 to 120 per 100,000 pregnancies. The prevalence rates of both hydatidiform mole and choriocarcinoma have declined over the past three decades in all groups, which may be due to better awareness of the condition leading to risk factor avoidance, dietary improvement and decreased overall birth rate.[1]
Hydatidiform Mole
- The worldwide prevalence of molar pregnancy varies according to geographic region, ranging from 1200 per 100,000 pregnancies in Indonesia, India, and Turkey to 100-200 per 100,000 pregnancies in Japan and China and 50 to 100 per 100,000 pregnancies in North America and Europe.[2]
Choriocarcinoma
- The reported incidence of choriocarcinoma, the most aggressive form of GTD, in the United States is about 2 to 7 per 100,000 pregnancies. The U.S. age-standardized (1960 World Population Standard) incidence rate of choriocarcinoma is about 0.18 per 100,000 women between the ages of 15 years and 49 years.
- The reported prevalence of choriocarcinoma varies widely according to the geographic region, and ranges from a low of 2 per 100,000 pregnancies in the United States to a high of 202 per 100,000 pregnancies in China.[3]
Risk Factors
Risk factors for gestational trophoblastic disease include the following:[4][5][6]
- Maternal age (younger than 20 and greater than 35 years of age- bimodal distribution, relative risk 1.1 to 11)
- History of hydatidiform mole (including positive family history- associated with a 1 % risk in subsequent pregnancies, 25 % with history of more than one prior hydatidiform mole)
- Low levels of carotene and vitamin A
- Blood groups A or AB
- Prior spontaneous abortions
- Multigravida women
Clinical Features
Gestational trophoblastic disease (GTD) may present with the following clinical features:[7][8]
- Vaginal bleeding
- Rapidly enlarging uterus
- Elevated human chorionic gonadotropin (hCG) levels
- Pelvic pain or sensation of pressure
- Anemia
- Hyperemesis gravidarum
- Hyperthyroidism (secondary to the homology between the beta-subunits of hCG and thyroid-stimulating hormone (TSH), which causes hCG to have weak TSH-like activity)
- Preeclampsia early in pregnancy
- Neurological symptoms (in cases of brain metastasis from choriocarcinoma)
- Anxiety
- Weight loss
- Insomnia
- Abdominal swelling
The following table outlines the variation in clinical presentations of molar pregnancy (complete, partial and invasive), choriocarcinoma, placental-site trophoblastic tumor (PSTT) and epithelioid trophoblastic tumor (ETT):[9][10][11][12][13]
| Clinical Features | Complete Hydatidiform Mole | Partial Hydatidiform Mole | Invasive Molar Pregnancy | Choriocarcinoma | Placental-site trophoblastic tumor (PSTT) and Epithelioid trophoblastic tumor (ETT) |
|---|---|---|---|---|---|
| Presenting Complaints |
|
|
|||
| Neoplastic Conversion |
|
|
|||
| Beta Human Chorionic Gonadotropin (Beta-hCG) baseline levels |
|
|
|
|
|
| History of Pregnancies |
|
|
|
|
|
| Metastatic Route | |||||
| Management |
Differential Diagnosis
Gestational trophoblastic disease must be differentiated from other causes of hyperthyroidism such as Grave's disease and toxic nodular goiter. The following table differentiates these causes of hyperthyroidism:
| Cause of thyrotoxicosis | TSH receptor antibodies | Thyroid US | Color flow Doppler | Radioactive iodine uptake/Scan | Other features |
|---|---|---|---|---|---|
| Graves' disease | + | Hypoechoic pattern | ? | Diffuse uptake | Ophthalmopathy, dermopathy, acropathy |
| Toxic nodular goiter | - | Multiple nodules | - | Hot nodules on thyroid scan | - |
| Toxic adenoma | - | Single nodule | - | Hot nodule | - |
| Subacute thyroiditis | - | Heterogeneous hypoechoic areas | Reduced/absent flow | ? | Neck pain, fever, and elevated inflammatory index |
| Painless thyroiditis | - | Hypoechoic pattern | Reduced/absent flow | ? | - |
| Amiodarone induced thyroiditis-Type 1 | - | Diffuse or nodular goiter | ?/Normal/? | ? but higher than in Type 2 | High urinary iodine |
| Amiodarone induced thyroiditis-Type 2 | - | Normal | Absent | ?/absent | High urinary iodine |
| Central hyperthyroidism | - | Diffuse or nodular goiter | Normal/? | ? | Inappropriately normal or high TSH |
| Trophoblastic disease | - | Diffuse or nodular goiter | Normal/? | ? | - |
| Factitious thyrotoxicosis | - | Variable | Reduced/absent flow | ? | Normal or decreased serum thyroglobulin |
| Struma ovarii | - | Variable | Reduced/absent flow | ? | Increased abdominal RAIU |
| Disease | Findings | |
|---|---|---|
| Thyroiditis | Direct chemical toxicity with inflammation | Amiodarone, sunitinib, pazopanib, axitinib, and other tyrosine kinase inhibitors may also be associated with a destructive thyroiditis.[14][15] |
| Radiation thyroiditis | Patients treated with radioiodine may develop thyroid pain and tenderness 5 to 10 days later, due to radiation-induced injury and necrosis of thyroid follicular cells and associated inflammation. | |
| Drugs that interfere with the immune system | Interferon-alfa is a well-known cause of thyroid abnormality. It mostly leads to the development of de novo antithyroid antibodies.[16] | |
| Lithium | Patients treated with lithium are at a high risk of developing painless thyroiditis and Graves' disease. | |
| Palpation thyroiditis | Manipulation of the thyroid gland during thyroid biopsy or neck surgery and vigorous palpation during the physical examination may cause transient hyperthyroidism. | |
| Exogenous and ectopic hyperthyroidism | Factitious ingestion of thyroid hormone | The diagnosis is based on the clinical features, laboratory findings, and 24-hour radioiodine uptake.[17] |
| Acute hyperthyroidism from a levothyroxine overdose | The diagnosis is based on the clinical features, laboratory findings, and 24-hour radioiodine uptake.[18] | |
| Struma ovarii | Functioning thyroid tissue is present in an ovarian neoplasm. | |
| Functional thyroid cancer metastases | Large bony metastases from widely metastatic follicular thyroid cancer cause symptomatic hyperthyroidism. | |
| Hashitoxicosis | It is an autoimmune thyroid disease that initially presents with hyperthyroidism and a high radioiodine uptake caused by TSH-receptor antibodies similar to Graves' disease. It is then followed by the development of hypothyroidism due to the infiltration of the thyroid gland with lymphocytes and the resultant autoimmune-mediated destruction of thyroid tissue, similar to chronic lymphocytic thyroiditis.[19] | |
| Toxic adenoma and toxic multinodular goiter | Toxic adenoma and toxic multinodular goiter are results of focal/diffuse hyperplasia of thyroid follicular cells independent of TSH regulation. Findings of single or multiple nodules are seen on physical examination or thyroid scan.[20] | |
| Iodine-induced hyperthyroidism | It is uncommon but can develop after an iodine load, such as administration of contrast agents used for angiography or computed tomography (CT), or iodine-rich drugs such as amiodarone. | |
| Trophoblastic disease and germ cell tumors | Thyroid-stimulating hormone and HCG have a common alpha-subunit and a beta-subunit with considerable homology. As a result, HCG has weak thyroid-stimulating activity and high titer HCG may mimic hyperthyroidism.[21] | |
Prognostic Factors and Survivorship
The prognosis for cure of patients with GTDs is good even when the disease has spread to distant organs, especially when only the lungs are involved. Therefore, the traditional TNM staging system has limited prognostic value. The probability of cure depends on the following:
- Histologic type (invasive mole or choriocarcinoma).
- Extent of spread of the disease/largest tumor size.
- Level of serum beta-hCG.
- Duration of disease from the initial pregnancy event to start of treatment.
- Number and specific sites of metastases.
- Nature of antecedent pregnancy.
- Extent of prior treatment.
Selection of treatment depends on these factors plus the patient’s desire for future pregnancies. The beta-hCG is a sensitive marker to indicate the presence or absence of disease before, during, and after treatment. Given the extremely good therapeutic outcomes of most of these tumors, an important goal is to distinguish patients who need less-intensive therapies from those who require more-intensive regimens to achieve a cure.
References
- ↑ Martin BH, Kim JH (January 1998). "Changes in gestational trophoblastic tumors over four decades. A Korean experience". J Reprod Med. 43 (1): 60–8. PMID 9475151.
- ↑ Steigrad SJ (December 2003). "Epidemiology of gestational trophoblastic diseases". Best Pract Res Clin Obstet Gynaecol. 17 (6): 837–47. PMID 14614884.
- ↑ Altieri A, Franceschi S, Ferlay J, Smith J, La Vecchia C (November 2003). "Epidemiology and aetiology of gestational trophoblastic diseases". Lancet Oncol. 4 (11): 670–8. PMID 14602247.
- ↑ Parazzini F, Mangili G, La Vecchia C, Negri E, Bocciolone L, Fasoli M (December 1991). "Risk factors for gestational trophoblastic disease: a separate analysis of complete and partial hydatidiform moles". Obstet Gynecol. 78 (6): 1039–45. PMID 1945204.
- ↑ Messerli ML, Lilienfeld AM, Parmley T, Woodruff JD, Rosenshein NB (October 1985). "Risk factors for gestational trophoblastic neoplasia". Am. J. Obstet. Gynecol. 153 (3): 294–300. PMID 2996354.
- ↑ Kumar N, Saxena YK, Rathi AK, Chitra R, Kumar P (October 2003). "Host and risk factors for gestational trophoblastic disease: a hospital-based analysis from India". Med. Sci. Monit. 9 (10): CR442–7. PMID 14523334.
- ↑ Sun SY, Melamed A, Goldstein DP, Bernstein MR, Horowitz NS, Moron AF, Maestá I, Braga A, Berkowitz RS (July 2015). "Changing presentation of complete hydatidiform mole at the New England Trophoblastic Disease Center over the past three decades: does early diagnosis alter risk for gestational trophoblastic neoplasia?". Gynecol. Oncol. 138 (1): 46–9. doi:10.1016/j.ygyno.2015.05.002. PMID 25969351.
- ↑ Seckl MJ, Sebire NJ, Berkowitz RS (August 2010). "Gestational trophoblastic disease". Lancet. 376 (9742): 717–29. doi:10.1016/S0140-6736(10)60280-2. PMID 20673583.
- ↑ "Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole - American Journal of Obstetrics & Gynecology".
- ↑ Dhanda S, Ramani S, Thakur M (2014). "Gestational trophoblastic disease: a multimodality imaging approach with impact on diagnosis and management". Radiol Res Pract. 2014: 842751. doi:10.1155/2014/842751. PMC 4122023. PMID 25126425.
- ↑ Milenković V, Lazović B (2011). "[Gestational trophoblastic disease--literature review]". Med. Pregl. 64 (3–4): 188–93. PMID 21905598.
- ↑ "academic.oup.com".
- ↑ Joneborg U, Marions L (2014). "Current clinical features of complete and partial hydatidiform mole in Sweden". J Reprod Med. 59 (1–2): 51–5. PMID 24597287.
- ↑ Lambert M, Unger J, De Nayer P, Brohet C, Gangji D (1990). "Amiodarone-induced thyrotoxicosis suggestive of thyroid damage". J. Endocrinol. Invest. 13 (6): 527–30. PMID 2258582.
- ↑ Ahmadieh H, Salti I (2013). "Tyrosine kinase inhibitors induced thyroid dysfunction: a review of its incidence, pathophysiology, clinical relevance, and treatment". Biomed Res Int. 2013: 725410. doi:10.1155/2013/725410. PMC 3824811. PMID 24282820.
- ↑ Vialettes B, Guillerand MA, Viens P, Stoppa AM, Baume D, Sauvan R, Pasquier J, San Marco M, Olive D, Maraninchi D (1993). "Incidence rate and risk factors for thyroid dysfunction during recombinant interleukin-2 therapy in advanced malignancies". Acta Endocrinol. 129 (1): 31–8. PMID 8351956.
- ↑ Cohen JH, Ingbar SH, Braverman LE (1989). "Thyrotoxicosis due to ingestion of excess thyroid hormone". Endocr. Rev. 10 (2): 113–24. doi:10.1210/edrv-10-2-113. PMID 2666114.
- ↑ Jha S, Waghdhare S, Reddi R, Bhattacharya P (2012). "Thyroid storm due to inappropriate administration of a compounded thyroid hormone preparation successfully treated with plasmapheresis". Thyroid. 22 (12): 1283–6. doi:10.1089/thy.2011.0353. PMID 23067331.
- ↑ Fatourechi V, McConahey WM, Woolner LB (1971). "Hyperthyroidism associated with histologic Hashimoto's thyroiditis". Mayo Clin. Proc. 46 (10): 682–9. PMID 5171000.
- ↑ Laurberg P, Pedersen KM, Vestergaard H, Sigurdsson G (1991). "High incidence of multinodular toxic goitre in the elderly population in a low iodine intake area vs. high incidence of Graves' disease in the young in a high iodine intake area: comparative surveys of thyrotoxicosis epidemiology in East-Jutland Denmark and Iceland". J. Intern. Med. 229 (5): 415–20. PMID 2040867.
- ↑ Oosting SF, de Haas EC, Links TP, de Bruin D, Sluiter WJ, de Jong IJ, Hoekstra HJ, Sleijfer DT, Gietema JA (2010). "Prevalence of paraneoplastic hyperthyroidism in patients with metastatic non-seminomatous germ-cell tumors". Ann. Oncol. 21 (1): 104–8. doi:10.1093/annonc/mdp265. PMID 19605510.