Imipramine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
Suicidality and Antidepressant DrugsSee full prescribing information for complete Boxed Warning.
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of imipramine hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Imipramine hydrochloride is not approved for use in pediatric patients
|
Overview
Imipramine is a Tricyclic antidepressant that is FDA approved for the {{{indicationType}}} of depression, nocturnal enuresis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include weight gain, bloating symptom, constipation, xerostomia, asthenia, dizziness, headache, somnolence, blurred vision, urinary retention, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Depression
- Hospitalized patients
- 100 mg orally per day in divided doses; may increase up to a max of 300 mg/day
- Outpatients
- 75 mg orally per day; may increase up to a max of 200 mg/day; usual maintenance dose, 50 to 150 mg/day
Nocturnal enuresis
- 25 mg orally at bedtime, may increase in 25 mg increments to max dose of 150mg at bedtime
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Imipramine in adult patients
Non–Guideline-Supported Use
There is limited information about Off-Label Non-Guideline-Supported Use of Imipramine in adult patients
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Nocturnal enuresis
- Children 6 to 12 y
- Initial, 25 mg orally 1 h before bedtime, may increase in 25 mg increments to max dose of 50 mg/d or 2.5 mg/kg/d
- Children over 12 y
- Initial, 25 mg orally 1 h before bedtime, may increase in 25 mg increments to max dose of 75 mg/d or 2.5 mg/kg/d
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Imipramine in pediatric patients
Non–Guideline-Supported Use
There is limited information about Off-Label Non-Guideline-Supported Use of Imipramine in pediatric patients
Contraindications
- Concomitant use of monoamine oxidase inhibiting compounds
- Acute recovery period after a myocardial infarction
- Hypersensitivity
Warnings
Suicidality and Antidepressant DrugsSee full prescribing information for complete Boxed Warning.
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of imipramine hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Imipramine hydrochloride is not approved for use in pediatric patients
|
Clinical Worsening and Suicide Risk
- Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
- The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table.
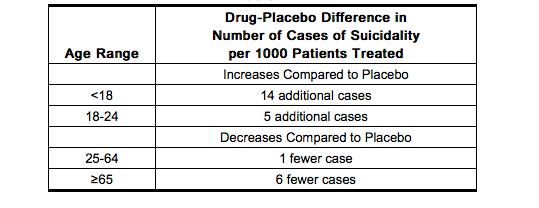
- No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
- It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
- All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
- The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
- Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms.
- Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for imipramine hydrochloride should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder
- A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that imipramine hydrochloride is not approved for use in treating bipolar depression.
Children
- A dose of 2.5 mg/kg/day of imipramine hydrochloride should not be exceeded in childhood. ECG changes of unknown significance have been reported in pediatric patients with doses twice this amount.
- Extreme caution should be used when this drug is given to:
- Patients with cardiovascular disease because of the possibility of conduction defects, arrhythmias, congestive heart failure, myocardial infarction, strokes, and tachycardia. These patients require cardiac surveillance at all dosage levels of the drug; patients with increased intraocular pressure, history of urinary retention, or history of narrow-angle glaucoma because of the drug’s anticholinergic properties; hyperthyroid patients or those on thyroid medication because of the possibility of cardiovascular toxicity;patients with a history of seizure disorder because this drug has been shown to lower the seizure threshold; patients receiving guanethidine, clonidine, or similar agents, since imipramine hydrochloride may block the pharmacologic effects of these drugs.
- Patients receiving methylphenidate hydrochloride. Since methylphenidate hydrochloride may inhibit the metabolism of imipramine, downward dosage adjustment of imipramine hydrochloride may be required when given concomitantly with methylphenidate hydrochloride.
- Imipramine may enhance the CNS depressant effects of alcohol. Therefore, it should be borne in mind that the dangers inherent in a suicide attempt or accidental overdosage with the drug may be increased for the patient who uses excessive amounts of alcohol.
- Since imipramine hydrochloride may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as operating an automobile or machinery, the patient should be cautioned accordingly.
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- Numbness, tingling, paresthesias of extremities, incoordination, ataxia, tremors; peripheral neuropathy; extrapyramidal symptoms ; seizures, alterations in EEG patterns; tinnitus.
Cardiovascular
- Orthostatic hypotension, hypertension, tachycardia, palpitation, myocardial infarction, arrhythmias, heart block, ECG changes, precipitation of congestive heart failure, stroke
Gastrointestinal
- Nausea and vomiting, anorexia, epigastric distress, diarrhea; peculiar taste, stomatitis, abdominal cramps, black tongue.
Hypersensitive Reactions
- Skin rash, petechiae, urticaria, itching, photosensitization, edema (general or of face and tongue); drug fever, cross-sensitivity with desipramine.
Miscellaneous
- Jaundice (simulating obstructive); altered liver function; weight gain or loss; perspiration; flushing; urinary frequency; drowsiness, dizziness, weakness and fatigue; headache; parotid swelling; alopecia; proneness to falling.
Postmarketing Experience
There is limited information about adverse reactions reported from postmarketing experience of Imipramine.
Drug Interactions
Drugs Metabolized by P450 2D6
- The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the Caucasian population (about 7% to 10% of Caucasians are so-called “poor metabolizers”); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African, and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8-fold increase in plasma AUC of the TCA).
- In addition, certain drugs inhibit the activity of this isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dose of TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, and paroxetine, inhibit P450 2D6, they may vary in the extent of inhibition. The extent to which SSRI-TCA interaction may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the co-administration of TCAs with any of the SSRIs and also in switching from one class to the other. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary).
- Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. Furthermore, whenever one of these other drugs is withdrawn from co-therapy, an increased dose of tricyclic antidepressant may be required. It is desirable to monitor TCA plasma levels whenever a TCA is going to be co-administered with another drug known to be an inhibitor of P450 2D6.
- The plasma concentration of imipramine may increase when the drug is given concomitantly with hepatic enzyme inhibitors (e.g., cimetidine, fluoxetine) and decrease by concomitant administration with hepatic enzyme inducers (e.g., barbiturates, phenytoin), and adjustment of the dosage of imipramine may therefore be necessary.
- In occasional susceptible patients or in those receiving anticholinergic drugs (including antiparkinsonism agents) in addition, the atropine-like effects may become more pronounced (e.g., paralytic ileus). Close supervision and careful adjustment of dosage is required when imipramine hydrochloride is administered concomitantly with anticholinergic drugs.
- Avoid the use of preparations, such as decongestants and local anesthetics, that contain any sympathomimetic amine (e.g., epinephrine, norepinephrine), since it has been reported that tricyclic antidepressants can potentiate the effects of catecholamines.
- Caution should be exercised when imipramine hydrochloride is used with agents that lower blood pressure. Imipramine hydrochloride may potentiate the effects of CNS depressant drugs.
- Patients should be warned that imipramine hydrochloride may enhance the CNS depressant effects of alcohol.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have yielded inconclusive results (see also Animal Pharmacology& Toxicology)
There have been no well-controlled studies conducted with pregnant women to determine the effect of imipramine hydrochloride on the fetus. However, there have been clinical reports of congenital malformations associated with the use of the drug. Although a causal relationship between these effects and the drug could not be established, the possibility of fetal risk from the maternal ingestion of imipramine hydrochloride cannot be excluded. Therefore, imipramine hydrochloride should be used in women who are or might become pregnant only if the clinical condition clearly justifies potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Imipramine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Imipramine during labor and delivery.
Nursing Mothers
- Limited data suggest that imipramine hydrochloride is likely to be excreted in human breast milk. As a general rule, a woman taking a drug should not nurse since the possibility exists that the drug may be excreted in breast milk and be harmful to the child.
Pediatric Use
- Safety and effectiveness in the pediatric population other than pediatric patients with nocturnal enuresis have not been established (see Box Warning and Warnings: Clinical Worsening and Suicide Risk). Anyone considering the use of imipramine hydrochloride in a child or adolescent must balance the potential risks with the clinical need.
- The safety and effectiveness of the drug as temporary adjunctive therapy for nocturnal enuresis in pediatric patients less than 6 years of age has not been established.
- The safety of the drug for long-term, chronic use as adjunctive therapy for nocturnal enuresis in pediatric patients 6 years of age or older has not been established; consideration should be given to instituting a drug-free period following an adequate therapeutic trial with a favorable response.
- A dose of 2.5 mg/kg/day should not be exceeded in childhood. ECG changes of unknown significance have been reported in pediatric patients with doses twice this amount.
Geriatic Use
- In the literature, there were four well-controlled, randomized, double-blind, parallel group comparison clinical studies done with imipramine in the elderly population. There was a total number of 651 subjects included in these studies. These studies did not provide a comparison to younger subjects. There were no additional adverse experiences identified in the elderly.
- Clinical studies of imipramine in the original application did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Post-marketing clinical experience has not identified differences in responses between the elderly and younger subjects. In general, dose selection for the elderly should be cautious, usually starting at the low end of the dosing range, reflecting greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. (See also Dosage and Administration:Adolescents and Geriatric Patients.) (See also Precautions: General)
Gender
There is no FDA guidance on the use of Imipramine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Imipramine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Imipramine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Imipramine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Imipramine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Imipramine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Imipramine Administration in the drug label.
Monitoring
There is limited information regarding Imipramine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Imipramine and IV administrations.
Overdosage
- Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic overdose. Therefore, hospital monitoring is required as soon as possible.
- Children have been reported to be more sensitive than adults to an acute overdosage of imipramine hydrochloride. An acute overdose of any amount in infants or young children, especially, must be considered serious and potentially fatal.
Manifestations
- These may vary in severity depending upon factors such as the amount of drug absorbed, the age of the patient, and the interval between drug ingestion and the start of treatment. Critical manifestations of overdose include cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic toxicity.
Other CNS manifestations may include drowsiness, stupor, ataxia, restlessness, agitation, hyperactive reflexes, muscle rigidity, athetoid and choreiform movements. Cardiac abnormalities may include tachycardia and signs of congestive failure. Respiratory depression, cyanosis, shock, vomiting, hyperpyrexia, mydriasis, and diaphoresis may also be present.
Management
- Obtain an ECG and immediately initiate cardiac monitoring. Protect the patient’s airway, establish an intravenous line and initiate gastric decontamination. A minimum of 6 hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during this period, extended monitoring is required. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient.
- Gastrointestinal Decontamination
- All patients suspected of tricyclic overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. Emesis is contraindicated.
- Cardiovascular
- A maximal limb-lead QRS duration of ≥0.10 seconds may be the best indication of the severity of the overdose. Intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH>7.60 or a pCO2<20 mmHg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium, or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
- In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in tricyclic poisoning.
- CNS
- In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
- Psychiatric Follow-up
- Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
- Pediatric Management
- The principles of management of child and adult overdosages are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
Pharmacology
Mechanism of Action
- The mechanism of action of imipramine hydrochloride is not definitely known. However, it does not act primarily by stimulation of the central nervous system. The clinical effect is hypothesized as being due to potentiation of adrenergic synapses by blocking uptake of norepinephrine at nerve endings. The mode of action of the drug in controlling childhood enuresis is thought to be apart from its antidepressant effect.
Structure
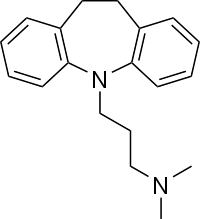
- Imipramine hydrochloride, the original tricyclic antidepressant, is a member of the dibenzazepine group of compounds. It is designated 5-[3-(Dimethylamino) propyl]-10, 11-dihydro-5H-dibenz [b,f]-azepine monohydrochloride. Structurally, it may be represented as follows:

- Imipramine hydrochloride USP is a white to off-white, odorless, or practically odorless crystalline powder. It is freely soluble in water and in alcohol, soluble in acetone, and insoluble in ether and in benzene. It melts at 170-174°C.
- Each tablet, for oral administration, contains 10 mg, 25 mg or 50 mg imipramine hydrochloride USP. Inactive ingredients for 10 mg include: corn starch, D & C Yellow #10 Aluminum Lake, FD & C Red #30 Aluminum Lake, hydroxypropyl cellulose, hypromellose, lactose (anhydrous), magnesium stearate, polyethylene glycol, povidone and titanium dioxide; for 25 mg: corn starch, D & C Yellow #10 Aluminum Lake, FD & C Blue #2 Aluminum Lake, FD & C Red #40 Aluminum Lake, hydroxypropyl cellulose, hypromellose, lactose (anhydrous), magnesium stearate, polyethylene glycol, povidone and titanium dioxide; for 50 mg: corn starch, D & C Yellow #10 Aluminum Lake, FD & C Blue #1 Aluminum Lake, FD & C Red #40 Aluminum Lake, hydroxypropyl cellulose, hypromellose, lactose (anhydrous), magnesium stearate, polyethylene glycol, povidone and titanium dioxide.
Pharmacodynamics
There is limited information information related to the pharmacodynamics of Imipramine.
Pharmacokinetics
There is limited information information related to the pharmacodynamics of Imipramine.
Nonclinical Toxicology
There is limited information regarding Imipramine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Imipramine Clinical Studies in the drug label.
How Supplied
- Imipramine hydrochloride tablets USP for oral administration are available as:
- 10 mg: Round, film-coated, yellow tablets, debossed GG on one side and 41 on the reverse side, and supplied as:
- NDC 0781-1762-31 bottles of 30
- NDC 0781-1762-01 bottles of 100
- NDC 0781-1762-10 bottles of 1000
- 25 mg: Round, film-coated, beige tablets, debossed GG on one side and 47 on the reverse side, and supplied as:
- NDC 0781-1764-31 bottles of 30
- NDC 0781-1764-01 bottles of 100
- NDC 0781-1764-10 bottles of 1000
- NDC 0781-1764-13 unit dose packages of 100
- 50 mg: Round, film-coated, green tablets, debossed GG on one side and 42 on the reverse side, and supplied as:
- NDC 0781-1766-31 bottles of 30
- NDC 0781-1766-01 bottles of 100
- NDC 0781-1766-10 bottles of 1000
- NDC 0781-1766-13 unit dose packages of 100
Storage
- Store at 20°-25°C (68°-77°F) (see USP Controlled Room Temperature).
- Dispense in a tight, light-resistant container.
Images
Drug Images
{{#ask: Page Name::Imipramine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Imipramine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or Actions
- Read the Medication Guide that comes with you or your family member’s antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines. Talk to your, or your family member’s, healthcare provider about:
- All risks and benefits of treatment with antidepressant medicines
- All treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
- Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- Thoughts about suicide or dying
- Attempts to commit suicide
- New or worse depression
- New or worse anxiety
- Feeling very agitated or restless
- Panic attacks
- Trouble sleeping (insomnia)
- New or worse irritability
- Acting aggressive, being angry, or violent
- Acting on dangerous impulses
- An extreme increase in activity and talking (mania)
- Other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
- Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
- This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
- 11-2008M
- 7203
- Sandoz Inc.
- Princeton, NJ 08540
Precautions with Alcohol
Alcohol-Imipramine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Imipramine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Imipramine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Heck, HA (June 1979). "Bioavailability of imipramine tablets relative to a stable isotope-labelled internal standard: increasing the power of bioavailability tests". Journal of Pharmacokinetics and Biopharmaceutics. 7 (3): 233–248. PMID 480146. Unknown parameter
|coauthors=ignored (help) - ↑ 2.0 2.1 2.2 2.3 "PRODUCT INFORMATION TOLERADE® (imipramine hydrochloride)". TGA eBusiness Services. PMIP Pty Ltd. 4 June 2013. Retrieved 16 October 2013.
{{#subobject:
|Page Name=Imipramine |Pill Name=Imipramine_4.jpg |Drug Name=Imipramine |Pill Ingred=Imipramine, starch, corn, d&c yellow no. 10, fd&c blue no. 1, fd&c red no. 40, hydroxypropyl cellulose, hypromelloses, anhydrous lactose, magnesium stearate, polyethylene glycol, povidone, titanium dioxide|+sep=; |Pill Imprint=GG;42 |Pill Dosage=50 mg |Pill Color=Green|+sep=; |Pill Shape=Round |Pill Size (mm)=8.00 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=0781-1766-31
}}
{{#subobject:
|Label Page=Imipramine |Label Name=IMIPRAMINE5.jpg
}}
{{#subobject:
|Label Page=Imipramine |Label Name=IMIPRAMINE6.jpg
}}
{{#subobject:
|Label Page=Imipramine |Label Name=IMIPRAMINE7.jpg
}}
{{#subobject:
|Label Page=Imipramine |Label Name=IMIPRAMINE8.jpg
}}
{{#subobject:
|Label Page=Imipramine |Label Name=IMIPRAMINE9.jpg
}}
{{#subobject:
|Label Page=Imipramine |Label Name=IMIPRAMINE10.jpg
}}
{{#subobject:
|Label Page=Imipramine |Label Name=Imipramine table.png
}}
|